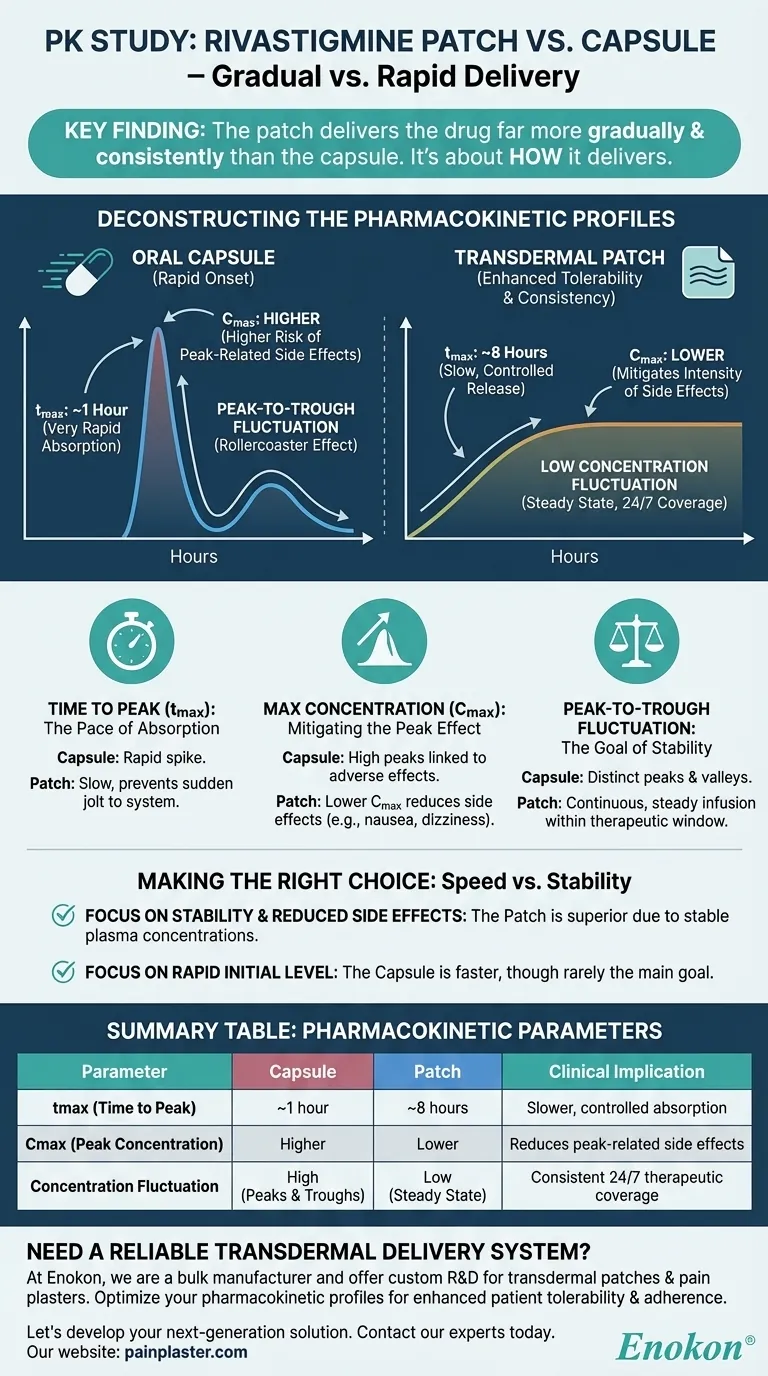
The primary finding of the pharmacokinetic study is that the rivastigmine patch delivers the drug far more gradually and consistently than the oral capsule. The patch reached its peak drug concentration in the blood (tmax) at a median of 8 hours, compared to just 1 hour for the capsule. This slower absorption resulted in a lower overall peak concentration (Cmax) and significantly less variation between the highest and lowest drug levels throughout the day.
The core difference is not about which method delivers more drug, but how it delivers it. The capsule creates a rapid spike, while the patch is engineered for a smooth, sustained, and stable therapeutic level, which has profound implications for patient tolerability.
Deconstructing the Pharmacokinetic Profiles
To understand the clinical impact, we must break down what these pharmacokinetic terms mean in practice. The data reveals two fundamentally different approaches to achieving a therapeutic effect.
Time to Peak Concentration (tmax): The Pace of Absorption
The tmax tells us how quickly the drug is absorbed into the bloodstream.
The capsule’s 1-hour tmax indicates a very rapid absorption process. This results in a fast onset of the drug's effects and its potential side effects.
In contrast, the patch’s 8-hour tmax demonstrates a slow, controlled release through the skin over many hours, preventing a sudden jolt to the system.
Maximum Concentration (Cmax): Mitigating the Peak Effect
The Cmax is the highest concentration the drug reaches in the body after a dose. High peaks are often linked to a higher incidence of adverse effects.
The study found the patch produces a lower Cmax. By avoiding the sharp spike associated with the capsule, the patch can reduce the intensity of peak-related side effects.
This is a critical advantage for drugs where side effects, such as nausea or dizziness, can limit a patient's ability to continue treatment.
Peak-to-Trough Fluctuation: The Goal of Stability
Perhaps the most important finding was the difference in concentration fluctuation, which is the "rollercoaster effect" of drug levels rising and falling between doses.
Oral capsules create distinct peaks and troughs. The drug level is high shortly after taking a dose and falls significantly before the next one is due.
The patch’s transdermal delivery smooths out these peaks and valleys. It provides a continuous, steady infusion of medication, keeping the drug concentration within the desired therapeutic window more consistently.
Understanding the Trade-offs: Speed vs. Stability
Neither delivery system is inherently superior; they are designed with different objectives in mind. The choice involves a clear trade-off between the speed of delivery and the stability of the drug concentration.
The Capsule's Profile: Rapid Onset
The primary feature of the capsule is its speed. The drug is absorbed quickly, which can be beneficial in situations where a rapid therapeutic effect is the highest priority.
However, this speed comes at the cost of stability. The sharp peaks increase the risk of side effects, and the subsequent troughs could potentially lead to periods of suboptimal therapeutic coverage.
The Patch's Profile: Enhanced Tolerability and Consistency
The patch prioritizes stability above all else. Its slow absorption and low Cmax are intentionally designed to improve the drug's tolerability.
By maintaining a steady plasma concentration, the patch helps ensure a consistent therapeutic effect around the clock, which is often crucial for managing chronic conditions like Alzheimer's disease. This smoother profile is the key reason it often causes fewer gastrointestinal side effects than the oral formulation.
Making the Right Choice for Your Goal
The study's findings provide a clear framework for selecting the appropriate rivastigmine formulation based on the primary therapeutic objective.
- If your primary focus is minimizing side effects and ensuring consistent therapy: The patch is the superior choice due to its stable plasma concentrations and lower peak exposure.
- If your primary focus is improving patient or caregiver adherence: The once-daily application of the patch often simplifies the medication regimen compared to multiple daily capsules.
- If a very rapid initial drug level is the main clinical priority: The capsule offers a significantly faster time to peak concentration, though this is rarely the main goal for this medication.
Ultimately, understanding these pharmacokinetic differences empowers you to select the delivery method that best aligns with the patient's clinical needs and therapeutic goals.
Summary Table:
| Pharmacokinetic Parameter | Rivastigmine Capsule | Rivastigmine Patch | Clinical Implication |
|---|---|---|---|
| Time to Peak (tmax) | ~1 hour | ~8 hours | Patch provides slower, controlled absorption. |
| Peak Concentration (Cmax) | Higher | Lower | Patch reduces risk of peak-related side effects. |
| Concentration Fluctuation | High (Peaks & Troughs) | Low (Steady State) | Patch ensures consistent 24/7 therapeutic coverage. |
Need a reliable transdermal delivery system for your drug product?
At Enokon, we are a bulk manufacturer of reliable transdermal patches and pain plasters. Our technical expertise is at your service for custom R&D and development, helping healthcare and pharma distributors and brands create formulations with optimized pharmacokinetic profiles for enhanced patient tolerability and adherence.
Let's develop your next-generation transdermal solution. Contact our experts today to discuss your project.
Visual Guide
Related Products
- Herbal Eye Protection Patch Eye Patch
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Asthma Cough and Pain Relief Patch for Adults and Kids
- Menthol Gel Pain Relief Patch
- Icy Hot Menthol Medicine Pain Relief Patch
People Also Ask
- What are the steps for properly using eye patches? Maximize Benefits for Your Delicate Eye Area
- When should a doctor be consulted regarding the use of this patch? Key Safety Guidelines
- How do eye patches enhance the effectiveness of eye creams? Boost Your Eye Care Routine
- What are the main benefits of using eye patches in a skincare routine? Revitalize Your Under-Eye Area
- Should under eye patches be applied before or after moisturizer? Optimize Your Skincare Routine














