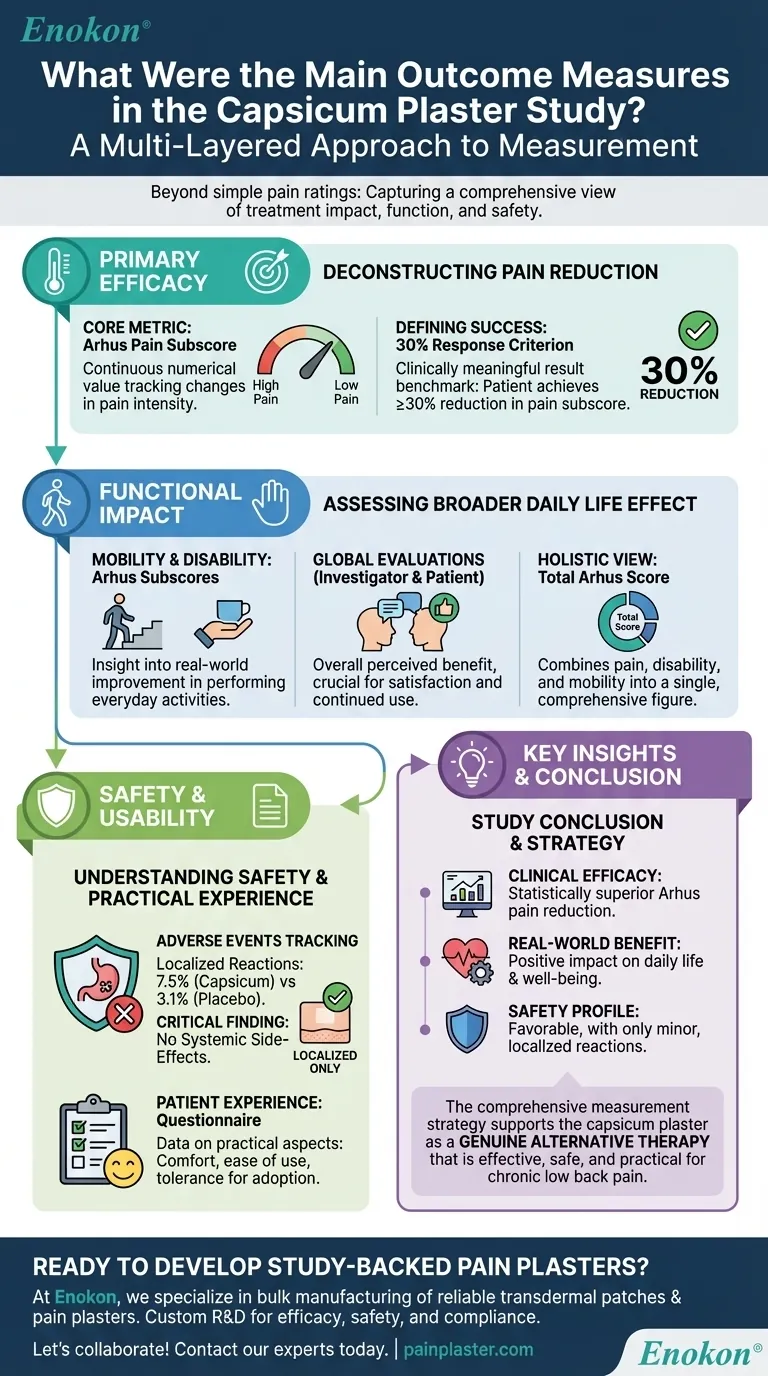
The primary outcome measures used in the capsicum plaster study were the compound pain subscore of the Arhus low back rating scale and a secondary response criterion defined as a 30% reduction in that pain score from the beginning to the end of the assessment period. These were supported by several additional measures to evaluate patient function, safety, and overall satisfaction.
The study's strength lies in its multi-layered approach to measurement. It moved beyond a simple pain rating to capture a comprehensive view of the treatment's impact, combining objective scores with functional improvements and patient-reported outcomes.
Deconstructing the Primary Efficacy Measures
To determine if the treatment worked, researchers needed specific, quantifiable metrics. They focused on two core indicators of pain reduction.
The Core Metric: The Arhus Pain Subscore
The central measure was a compound pain subscore derived from the Arhus low back rating scale. This provided a continuous numerical value to track changes in pain intensity over the course of the study.
Defining Success: The 30% Response Criterion
To translate the numerical score into a clinically meaningful result, a secondary measure was used. A patient was considered a "responder" if they experienced at least a 30% reduction in their pain subscore, providing a clear benchmark for successful treatment.
Assessing the Broader Impact on Patient Function
A treatment's true value is measured not just by pain reduction, but by its effect on a patient's daily life. The study incorporated several measures to capture this broader impact.
Beyond Pain: Mobility and Disability
The study also utilized the disability and mobility restriction subscores from the Arhus scale. These metrics assessed how the treatment affected a patient's ability to perform everyday activities, offering insight into real-world functional improvement.
The Subjective View: Global Efficacy Evaluations
Both the investigator and the patient provided a global evaluation of the treatment's effectiveness. This captured the overall perceived benefit, which is a critical factor in patient satisfaction and continued use of a therapy.
A Holistic View: The Total Arhus Score
Finally, the total score of the Arhus scale was considered. This combines the individual subscores for pain, disability, and mobility into a single, comprehensive figure representing the patient's overall condition.
Understanding the Safety and Usability Profile
No evaluation is complete without considering the potential downsides and the practical experience of using the treatment.
Tracking Adverse Events
Safety was a key concern. The study meticulously tracked adverse events, noting that local drug reactions (like skin irritation) occurred in 7.5% of capsicum plaster users compared to 3.1% of placebo users.
A Critical Safety Finding
Crucially, researchers observed no systemic side-effects. This finding is vital, as it positions the plaster as a localized treatment without broader, internal health risks.
The Patient Experience
The study also included a patient questionnaire on plaster use and tolerance evaluations. This gathered data on the practical aspects of the treatment, such as comfort and ease of use, which influence whether a patient would realistically adopt it.
Key Insights from the Measurement Strategy
The choice of outcome measures directly supports the study's conclusion that the capsicum plaster is a "genuine alternative therapy."
- If your primary focus is clinical efficacy: The statistically superior reduction in the Arhus pain subscore provides the core, objective evidence of the plaster's effectiveness against pain.
- If your primary focus is real-world patient benefit: The inclusion of mobility, disability, and global assessment scores demonstrates the treatment's positive impact on a patient's daily life and well-being.
- If your primary focus is safety: The detailed tracking of adverse events confirms a favorable safety profile, with only minor, localized reactions and no systemic side-effects.
This comprehensive measurement strategy allowed the researchers to build a robust case for the capsicum plaster as an effective, safe, and practical treatment for chronic low back pain.
Summary Table:
| Category | Key Outcome Measures | Purpose |
|---|---|---|
| Primary Efficacy | Arhus Low Back Rating Scale (Pain Subscore) | Quantify reduction in pain intensity |
| Secondary Efficacy | 30% Reduction in Pain Score (Responder Criterion) | Define clinically meaningful success |
| Functional Impact | Disability & Mobility Subscores (Arhus Scale) | Assess improvement in daily activities |
| Patient Perspective | Global Efficacy Evaluations (Investigator & Patient) | Capture perceived treatment benefit |
| Safety Profile | Adverse Events (Local Reactions, Systemic Effects) | Evaluate treatment tolerability and risks |
| Usability | Patient Questionnaire on Plaster Use | Gauge practical adoption and comfort |
Ready to develop a transdermal patch or pain plaster with proven clinical outcomes?
At Enokon, we specialize in bulk manufacturing of reliable, study-backed transdermal patches and pain plasters for healthcare and pharmaceutical distributors and brands. Our technical expertise ensures custom R&D and development tailored to your specific therapeutic needs—whether you're focused on efficacy, safety, or patient compliance.
Let’s collaborate to create a product that stands up to rigorous clinical scrutiny. Contact our experts today to discuss your project requirements.
Visual Guide
Related Products
- Heat Relief Capsicum Patch for Lower Back Pain Relief
- Capsaicin Chili Medicated Pain Relief Patches
- Natural Herbal Wormwood Patch Pain Plaster
- Mugwort Wormwood Pain Relief Patch for Neck Pain
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
People Also Ask
- What is the Capsicum HOT PATCH Adhesive Patch used for? Relieve Pain Naturally with Capsaicin
- How quickly does the Deep Heat Pain Relief Back Patch activate and how long does it provide warmth? Get 16-Hour Relief
- What are the key features of the Deep Heat Pain Relief Back Patch? Get Up to 16 Hours of Drug-Free Relief
- How does the active ingredient Capsicum in the Heat Patch work? A Dual-Action Path to Pain Relief
- How should the Capsicum HOT PATCH Adhesive Patch be stored? Best Practices for Safety & Effectiveness















