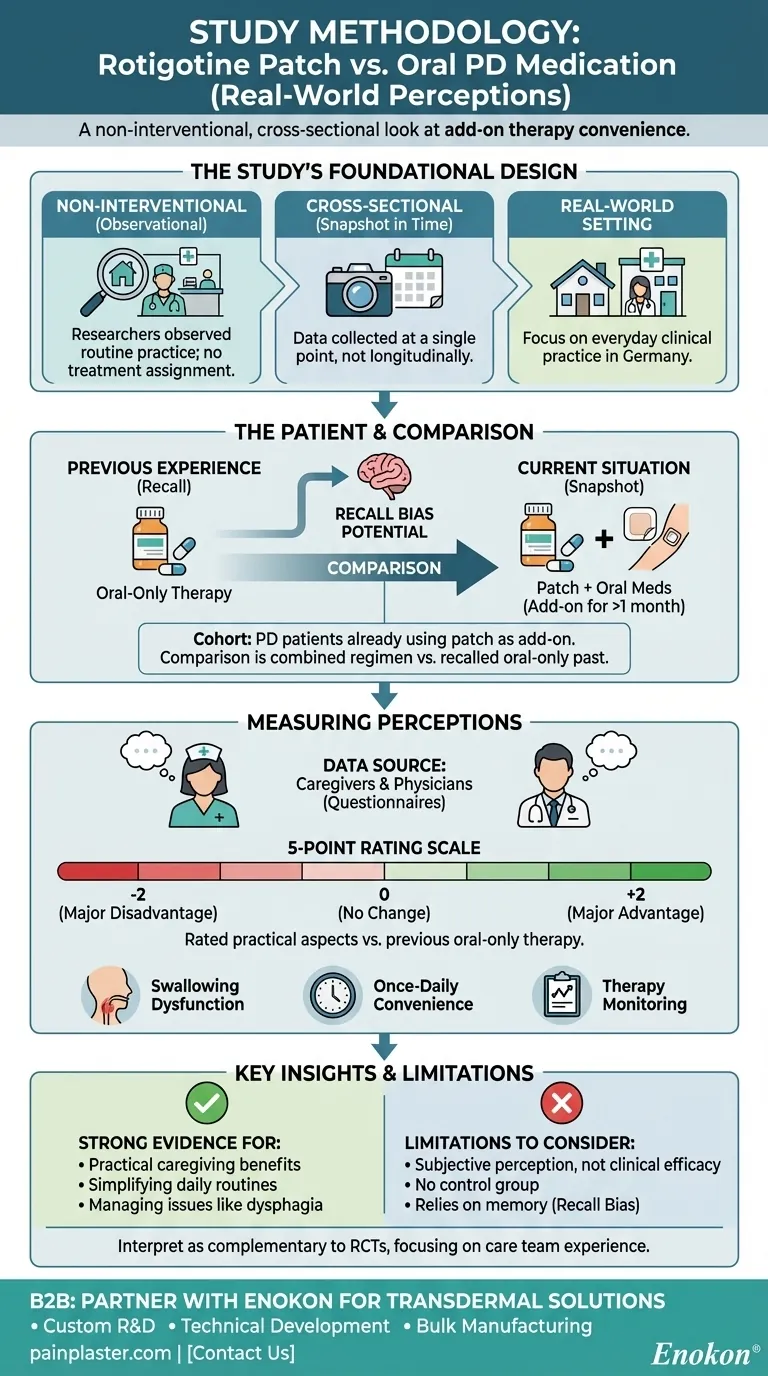
The study compared the rotigotine transdermal patch to oral medication using a cross-sectional, non-interventional design. This approach was conducted in routine clinical practice in Germany, focusing on patients with Parkinson's Disease (PD) who already had the patch prescribed as an add-on to their existing oral therapy for at least one month. Data was collected via questionnaires completed by caregivers and physicians, who rated their perceptions on a 5-point scale.
This study was designed to capture real-world perceptions of treatment convenience and management, not to measure objective clinical efficacy. Its value lies in understanding the practical, everyday experiences of the care team when a transdermal patch is added to a treatment regimen.

The Study's Foundational Design
To understand the results, we must first understand the methodology. The study's design choices directly influence how its findings should be interpreted.
A Non-Interventional, "Real-World" Approach
The study was non-interventional, meaning researchers did not assign treatments. They simply observed patients who were already receiving the rotigotine patch as part of their normal care.
It was also cross-sectional, which means it captured data at a single point in time—a snapshot of the current situation. This differs from a longitudinal study that would follow patients over an extended period.
The goal was to gather data from "routine clinical practice" to see how the treatment is perceived in a typical environment, outside the strict confines of a traditional clinical trial.
The Specific Patient Group
The study included a very specific cohort of patients. All participants had a documented PD diagnosis, required care, and had been using the rotigotine transdermal patch as an add-on therapy for at least one month.
This "add-on" status is a critical detail. The comparison was not between the patch versus oral medication, but rather the experience of a combined regimen (patch + oral meds) compared to the previous experience of oral-only medication.
How Perceptions Were Measured
The core of the study was subjective data collection from those administering care, not objective clinical measurements.
Relying on Caregiver and Physician Input
The primary sources of data were caregivers/nurses and physicians. They were chosen because the study's objective was to understand perceptions related to the practical aspects of treatment and caregiving.
The Questionnaire Framework
A structured questionnaire was used to assess various aspects of treatment. These included practical concerns like:
- Swallowing dysfunction (dysphagia)
- Nausea and vomiting
- Ease of therapy monitoring
- Convenience of once-daily application
- Independence from meal times
- Ability to apply to a sleeping patient
The questionnaire also included questions about overall caregiving effort for caregivers and other clinical aspects for physicians.
The 5-Point Rating Scale
Each item on the questionnaire was rated on a 5-point scale, ranging from -2 (major disadvantage) to +2 (major advantage).
This scale explicitly asked assessors to compare the current situation (with the patch) to their memory of the previous situation (oral-only therapy). A score of 0 indicated no perceived change.
Understanding the Method's Limitations
This study's "real-world" design provides valuable insights, but it's essential to recognize its inherent trade-offs and limitations.
Perception vs. Clinical Efficacy
The study measures subjective perception, not objective clinical outcomes. It tells us how caregivers and doctors feel about the treatment's convenience and side-effect profile, not whether it improved motor scores on a standardized scale like the UPDRS.
The Lack of a Control Group
As a non-interventional study, there was no randomized, concurrent control group. This makes it impossible to definitively rule out other factors or biases that may have influenced the assessors' positive perceptions.
Potential for Recall Bias
The comparison relied on the assessors' memory of what the oral-only therapy was like. This recall bias means their recollection of past difficulties might not be perfectly accurate, potentially influencing their rating of the current therapy.
How to Interpret These Findings
Use this methodological context to apply the study's conclusions appropriately.
- If your primary focus is on practical caregiving and treatment administration: This study provides strong evidence that care teams perceive the addition of a rotigotine patch as beneficial for simplifying daily routines and managing issues like swallowing problems.
- If your primary focus is on rigorous clinical effectiveness: View this study as complementary evidence. It supports the usability of the patch but should be weighed alongside randomized controlled trials (RCTs) that measure objective motor and non-motor symptom improvements.
- If your primary focus is on the patient experience: Acknowledge that this study reflects the valuable perspective of the care team, but it does not directly capture patient-reported outcomes or satisfaction.
Understanding the how and why of a study's design is the critical first step to accurately applying its conclusions to your professional context.
Summary Table:
| Study Aspect | Method Used |
|---|---|
| Design Type | Cross-sectional, Non-interventional |
| Data Source | Questionnaires from caregivers & physicians |
| Rating Scale | 5-point scale (-2 to +2) |
| Comparison | Patch + Oral Meds vs. Recall of Oral-Only Therapy |
| Primary Focus | Perceptions of treatment convenience & management |
Need a reliable transdermal patch for your Parkinson's Disease or pain management portfolio?
This study highlights the real-world value of transdermal delivery. As Enokon, a bulk manufacturer of reliable transdermal patches and pain plasters, we partner with healthcare and pharma distributors and brands to bring these benefits to market.
Benefit from our expertise:
- Custom R&D: We tailor patch formulations to meet specific clinical and patient needs.
- Technical Development: Leverage our experience to develop effective and user-friendly products.
- Bulk Manufacturing: Scale production with consistent, high-quality output.
Contact our team today to discuss how we can support your custom transdermal patch development.
Visual Guide
Related Products
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Herbal Eye Protection Patch Eye Patch
- Heating Pain Relief Patches for Menstrual Cramps
- Icy Hot Menthol Medicine Pain Relief Patch
- Menthol Gel Pain Relief Patch
People Also Ask
- How does the Deep Heat Back Patch work? A Drug-Free Solution for Targeted Pain Relief
- How does capsaicin work in the medicated heat patch? The Science Behind Pain Relief
- How quickly does the Deep Heat Pain Relief Back Patch activate and how long does it provide warmth? Get 16-Hour Relief
- What are the key features of the Deep Heat Pain Relief Back Patch? Get Up to 16 Hours of Drug-Free Relief
- How do Deep Heat Pain Relief Patches provide pain relief? Discover the Drug-Free Mechanism














