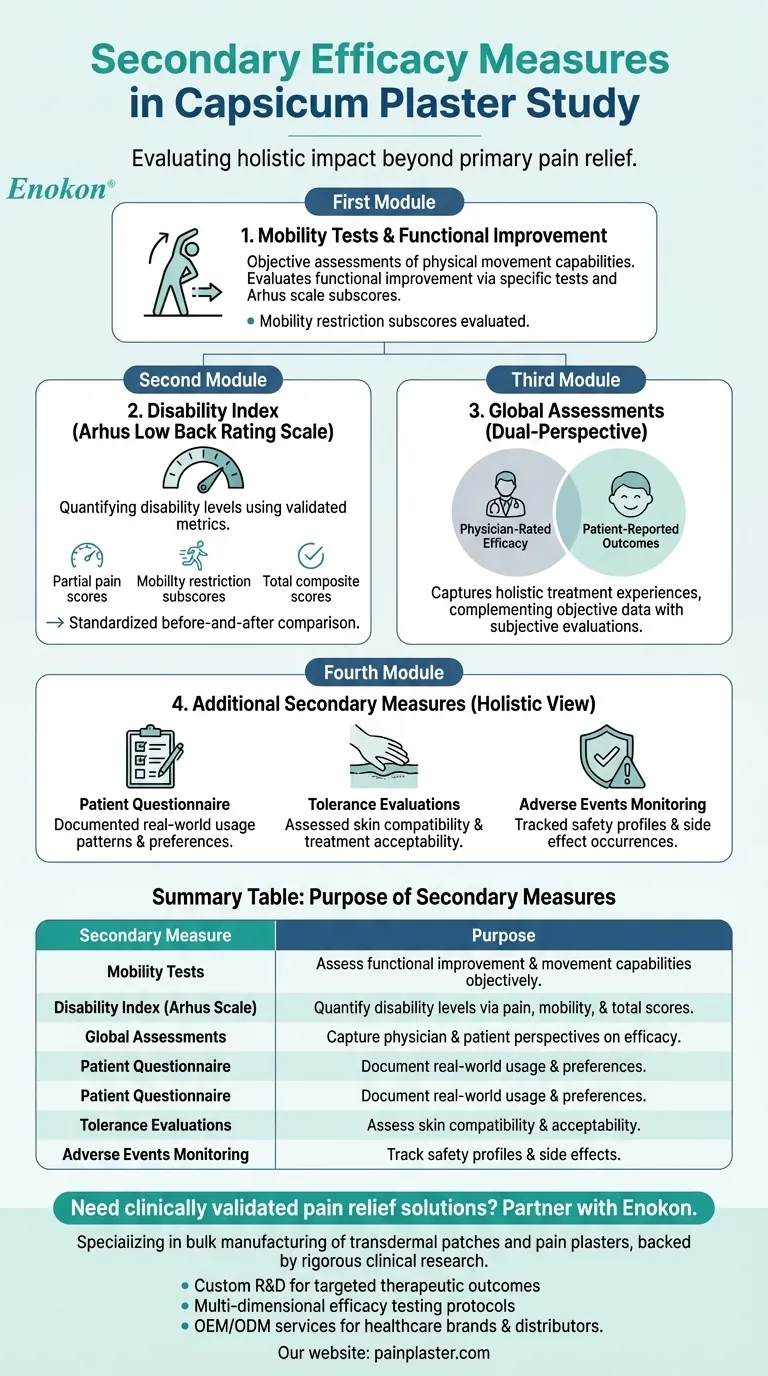
The secondary efficacy measures in the capsicum plaster study were designed to comprehensively evaluate its impact beyond primary pain relief. These measures included mobility tests, a disability index based on the Arhus low back rating scale, and global assessments by both physicians and patients. Additional evaluations covered partial pain scores, mobility restriction subscores, total Arhus scale scores, adverse events, patient questionnaires on plaster use, and tolerance assessments. This multi-faceted approach ensured a holistic understanding of the plaster's therapeutic effects and patient experiences.

Key Points Explained:
-
Mobility Tests
- These assessments measured the plaster's impact on patients' physical movement capabilities, providing objective data on functional improvement.
- Mobility restrictions were specifically evaluated through subscores within the Arhus rating system.
-
Disability Index (Arhus Low Back Rating Scale)
- The study utilized this validated scale to quantify disability levels, with particular attention to:
- Partial pain scores
- Mobility restriction subscores
- Total composite scores
- This provided standardized metrics for comparing functional limitations before and after treatment.
- The study utilized this validated scale to quantify disability levels, with particular attention to:
-
Global Assessments
- Dual-perspective evaluations were conducted:
- Physician-rated efficacy
- Patient-reported outcomes
- These subjective measures complemented objective data, capturing holistic treatment experiences.
- Dual-perspective evaluations were conducted:
-
Additional Secondary Measures
- Patient Questionnaire: Documented real-world plaster usage patterns and preferences
- Tolerance Evaluations: Assessed skin compatibility and treatment acceptability
- Adverse Events Monitoring: Tracked safety profiles and side effect occurrences
The study's comprehensive secondary measures framework allowed researchers to evaluate not just whether the plaster worked, but how it improved patients' quality of life and functional capacity. Have you considered how such multi-dimensional assessment approaches might influence your purchasing decisions for therapeutic products? These methodologies reveal the quiet but crucial ways clinical research shapes effective pain management solutions.
Summary Table:
| Secondary Measure | Purpose |
|---|---|
| Mobility Tests | Assess functional improvement and movement capabilities objectively. |
| Disability Index (Arhus Scale) | Quantify disability levels via pain scores, mobility subscores, and totals. |
| Global Assessments | Capture physician-rated efficacy and patient-reported outcomes. |
| Patient Questionnaire | Document real-world usage patterns and preferences. |
| Tolerance Evaluations | Assess skin compatibility and treatment acceptability. |
| Adverse Events Monitoring | Track safety profiles and side effects. |
Need clinically validated pain relief solutions?
At Enokon, we specialize in bulk manufacturing of transdermal patches and pain plasters, backed by rigorous clinical research. Our capsicum-based formulations are designed to deliver measurable improvements in mobility, pain relief, and quality of life—just as demonstrated in this study.
Why partner with us?
- Custom R&D for targeted therapeutic outcomes
- Multi-dimensional efficacy testing protocols
- OEM/ODM services for healthcare brands and distributors
Contact our team today to discuss how our evidence-based solutions can enhance your product line.
Visual Guide
Related Products
- Heat Relief Capsicum Patch for Lower Back Pain Relief
- Capsaicin Chili Medicated Pain Relief Patches
- Natural Herbal Wormwood Patch Pain Plaster
- Far Infrared Knee Pain Patch Heat Patches for Pain Relief
- Mugwort Wormwood Pain Relief Patch for Neck Pain
People Also Ask
- What should be done if a dose of the Capsicum HOT PATCH Adhesive Patch is missed? Follow These Steps for Safe Use
- What precautions should be taken when using the Capsicum HOT PATCH Adhesive Patch? Essential Safety Tips
- How does the Deep Heat Back Patch work? A Drug-Free Solution for Targeted Pain Relief
- What is the Capsicum HOT PATCH Adhesive Patch used for? Relieve Pain Naturally with Capsaicin
- What are the possible side effects of the Capsicum HOT PATCH Adhesive Patch? Risks & Safety Tips
















