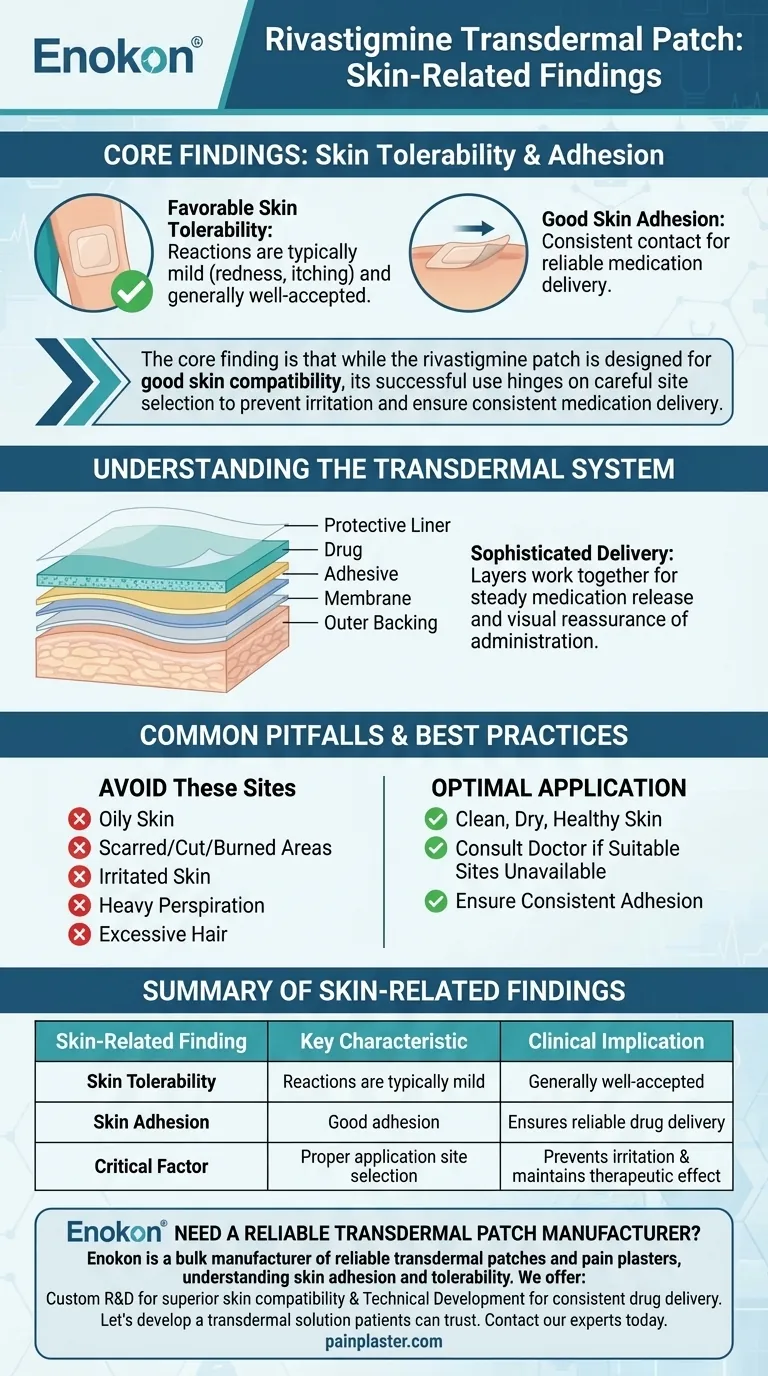
In clinical evaluation, the rivastigmine transdermal patch demonstrated both good skin adhesion and a favorable skin tolerability profile. The majority of application-site reactions observed were mild in severity, indicating the patch is generally well-accepted by the skin.
The core finding is that while the rivastigmine patch is designed for good skin compatibility, its successful use hinges on careful site selection to prevent irritation and ensure consistent medication delivery.
The Core Findings on Skin Interaction
The primary concerns for any transdermal system are how well it stays on and how the skin reacts to it. The rivastigmine patch performed well on both fronts.
Favorable Tolerability Profile
Studies found that skin reactions at the application site were typically mild. This suggests that for most patients, issues like redness or itching were not severe enough to discontinue treatment.
Reliable Skin Adhesion
The patch also showed good skin adhesion. This is a critical factor, as consistent contact with the skin is necessary for the medication to be absorbed correctly and deliver its intended therapeutic effect.
Understanding the Transdermal System
The patch is a sophisticated drug delivery system with several components that interact with the skin. Understanding them clarifies why application site matters so much.
Key Components of the Patch
A transdermal patch is composed of several layers, including a protective liner, the drug itself, an adhesive to stick to the skin, a membrane to control the release rate, and an outer backing. Each component is engineered to work together to deliver medication steadily over time.
The Goal of Transdermal Delivery
This system is designed to simplify the drug regimen and provide visual reassurance that the medication is being administered. By bypassing the digestive system, it can also reduce certain side effects and improve patient compliance.
Common Pitfalls and Best Practices for Application
While the patch is generally well-tolerated, its effectiveness and safety depend entirely on proper application. Avoiding common mistakes is key to preventing skin issues.
Skin Conditions to Avoid
The patch should never be applied to compromised skin. This includes areas that are oily, scarred, cut, burned, or already irritated. It is also critical to avoid areas of heavy perspiration or excessive hair, as these can interfere with adhesion.
Managing Unsuitable Skin Sites
If recommended application locations are consistently unsuitable due to skin conditions, it is important not to force it. In these cases, you should consult a doctor to discuss alternative adhesive support or other options to ensure treatment continuity without harming the skin.
Making the Right Choice for Skin Health and Efficacy
Proper application is the key to balancing the patch's benefits with potential skin reactions.
- If your primary focus is maximizing treatment efficacy: Ensure the patch is applied to clean, dry, and healthy skin to maintain the good adhesion necessary for consistent drug delivery.
- If your primary focus is minimizing skin irritation: Meticulously avoid applying the patch to any compromised or unsuitable skin areas and seek medical advice if appropriate sites are unavailable.
Ultimately, correct application technique is the single most important factor in successfully using the rivastigmine patch.
Summary Table:
| Skin-Related Finding | Key Characteristic | Clinical Implication |
|---|---|---|
| Skin Tolerability | Reactions are typically mild (e.g., redness, itching) | Generally well-accepted by the skin for most patients |
| Skin Adhesion | Demonstrates good adhesion to the skin | Ensures consistent contact for reliable drug delivery |
| Critical Factor | Proper application site selection | Prevents irritation and maintains therapeutic effect |
Need a reliable transdermal patch manufacturer?
As Enokon, a bulk manufacturer of reliable transdermal patches and pain plasters for healthcare and pharma distributors, we understand the critical importance of skin adhesion and tolerability. Our technical expertise ensures your product is designed for optimal performance and patient comfort.
We can help you with:
- Custom R&D to develop patches with superior skin compatibility.
- Technical Development focused on consistent drug delivery and reliable adhesion.
Let's develop a transdermal solution that patients can trust. Contact our experts today to discuss your project requirements.
Visual Guide
Related Products
- Herbal Eye Protection Patch Eye Patch
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Heating Pain Relief Patches for Menstrual Cramps
- Menthol Gel Pain Relief Patch
People Also Ask
- Can under eye patches smooth fine lines and wrinkles? Hydrate & Plump for Youthful Skin
- How quickly can you see results from using under eye patches? Instant Brightening & Long-Term Benefits
- What are the main benefits of using eye patches in a skincare routine? Revitalize Your Under-Eye Area
- Should under eye patches be applied before or after moisturizer? Optimize Your Skincare Routine
- When should a doctor be consulted regarding the use of this patch? Key Safety Guidelines















