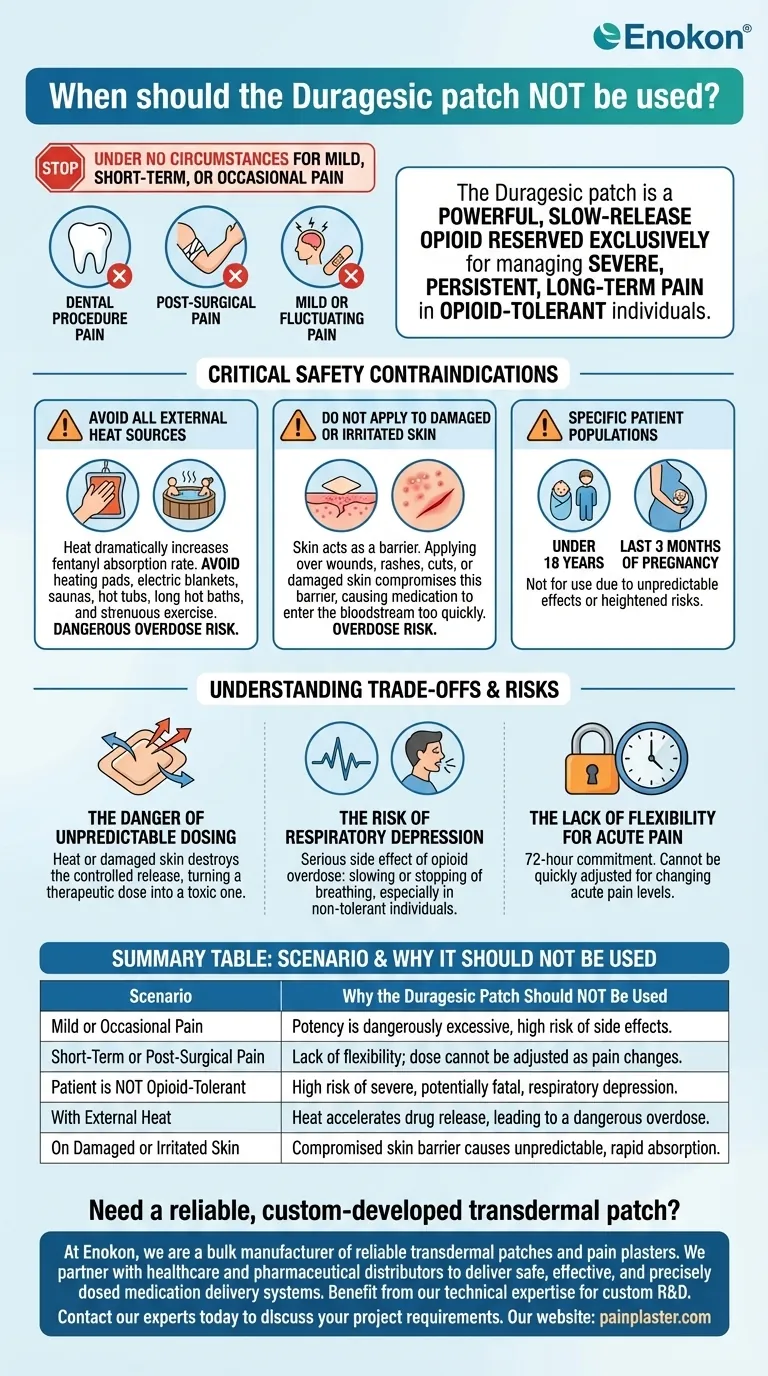
Under no circumstances should the Duragesic (fentanyl) patch be used to treat mild, occasional, or short-term pain, such as the pain following a dental procedure or surgery. This medication is a powerful, slow-release opioid reserved exclusively for managing severe, persistent, long-term pain in individuals who are already tolerant to opioid therapy. Using it outside of this specific context creates a significant risk of a life-threatening overdose.
The core principle is that the Duragesic patch is not a flexible, "as-needed" pain reliever. It is a potent, long-term medication designed for a very specific and serious medical situation: constant, severe chronic pain that is unresponsive to other treatments.

The Intended Use: A Tool for Severe, Chronic Pain
To understand when not to use the Duragesic patch, it's essential to understand its single, intended purpose. It is engineered to deliver a continuous, high dose of fentanyl over a 72-hour period to manage unrelenting pain.
Not for Mild or Occasional Pain
The strength of this medication is dangerously excessive for pain that is not severe. Using it for headaches, minor injuries, or pain that comes and goes introduces an unnecessary risk of serious side effects.
Not for Short-Term or Post-Surgical Pain
Acute pain, like that experienced after surgery, fluctuates and requires medication that can be adjusted quickly. A slow-release patch cannot be easily stopped or tailored, making it the wrong tool for managing pain that is expected to resolve in a few days or weeks.
Reserved for Opioid-Tolerant Patients
A critical safety requirement is that patients must be opioid-tolerant before starting this patch. This means their body is already accustomed to taking strong opioid medications regularly. Applying this patch to someone who is not opioid-tolerant can cause severe or fatal respiratory depression.
Critical Safety Contraindications
Beyond its intended use, several absolute rules must be followed to prevent a sudden, accidental overdose. These rules focus on preventing the patch from releasing the medication too quickly.
Avoid All External Heat Sources
Heat dramatically increases the rate at which fentanyl is absorbed from the patch into your bloodstream. This can quickly lead to a dangerous overdose.
You must not apply heat near the patch, which includes using heating pads, electric blankets, saunas, hot tubs, or taking long hot baths. You must also avoid activities that raise your body temperature, such as strenuous exercise.
Do Not Apply to Damaged or Irritated Skin
The skin acts as a barrier that controls the medication's release. Applying the patch over wounds, rashes, cuts, or any form of broken or damaged skin compromises this barrier. This allows the medication to enter the bloodstream much faster than intended, creating a serious overdose risk.
Use in Specific Patient Populations
The Duragesic patch should not be used in certain groups due to unpredictable effects or heightened risks. This includes individuals under 18 years of age and women during the last three months of pregnancy.
Understanding the Trade-offs and Risks
Using any powerful medication involves balancing benefits and risks. With the Duragesic patch, the risks are severe if used improperly.
The Danger of Unpredictable Dosing
The primary risk is losing control over the dose. The patch's design is based on a predictable, slow release through healthy skin at a normal body temperature. Applying heat or using it on broken skin destroys this controlled system, turning a therapeutic dose into a toxic one.
The Risk of Respiratory Depression
The most serious side effect of an opioid overdose is respiratory depression—the slowing or stopping of breathing. This is why the patch is so dangerous for short-term pain or for people who are not accustomed to strong opioids. Their bodies are not prepared for its potent effects on the central nervous system.
The Lack of Flexibility for Acute Pain
The patch is a 72-hour commitment. It cannot be quickly adjusted if pain subsides or if side effects become problematic. This lack of flexibility makes it fundamentally unsuitable for the changing nature of acute pain.
How to Apply This to Your Pain Management
Understanding these limitations is key to using this medication safely. Your decision, guided by a healthcare professional, must align with the medication's intended purpose.
- If your primary focus is managing pain after a recent surgery or injury: You need a faster-acting, more flexible medication that can be adjusted as your pain improves.
- If your primary focus is treating mild, intermittent, or "as-needed" pain: The Duragesic patch is dangerously inappropriate and potent for this situation.
- If your primary focus is managing diagnosed, severe, and persistent chronic pain: This is the only scenario where this patch may be considered, and it must be under the strict supervision of a doctor who has confirmed you are opioid-tolerant.
Knowing when not to use a powerful tool is just as important as knowing when to use it.
Summary Table:
| Scenario | Why the Duragesic Patch Should NOT Be Used |
|---|---|
| Mild or Occasional Pain | Potency is dangerously excessive, high risk of side effects. |
| Short-Term or Post-Surgical Pain | Lack of flexibility; dose cannot be adjusted as pain changes. |
| Patient is NOT Opioid-Tolerant | High risk of severe, potentially fatal, respiratory depression. |
| With External Heat (e.g., heating pads, hot tubs) | Heat accelerates drug release, leading to a dangerous overdose. |
| On Damaged or Irritated Skin | Compromised skin barrier causes unpredictable, rapid absorption. |
Need a reliable, custom-developed transdermal patch for your specific therapeutic needs?
At Enokon, we are a bulk manufacturer of reliable transdermal patches and pain plasters. We partner with healthcare and pharmaceutical distributors and brands to deliver safe, effective, and precisely dosed medication delivery systems.
Benefit from our technical expertise for custom R&D and development to ensure your product meets the highest safety and efficacy standards.
Contact our experts today to discuss your project requirements.
Visual Guide
Related Products
- Heating Pain Relief Patches for Menstrual Cramps
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Asthma Cough and Pain Relief Patch for Adults and Kids
- Menthol Gel Pain Relief Patch
- Icy Hot Menthol Medicine Pain Relief Patch
People Also Ask
- How often should HRT patches be changed? Optimize Your Hormone Therapy Schedule
- How quickly does the Deep Heat Pain Relief Back Patch activate and how long does it provide warmth? Get 16-Hour Relief
- Can heating pads be used with pain patches? Risks & Safety Tips
- What types of pain can the Deep Heat Pain Relief Back Patch be used for? Targeted Relief for Muscles & Joints
- What are the key features of the Deep Heat Pain Relief Back Patch? Get Up to 16 Hours of Drug-Free Relief

















