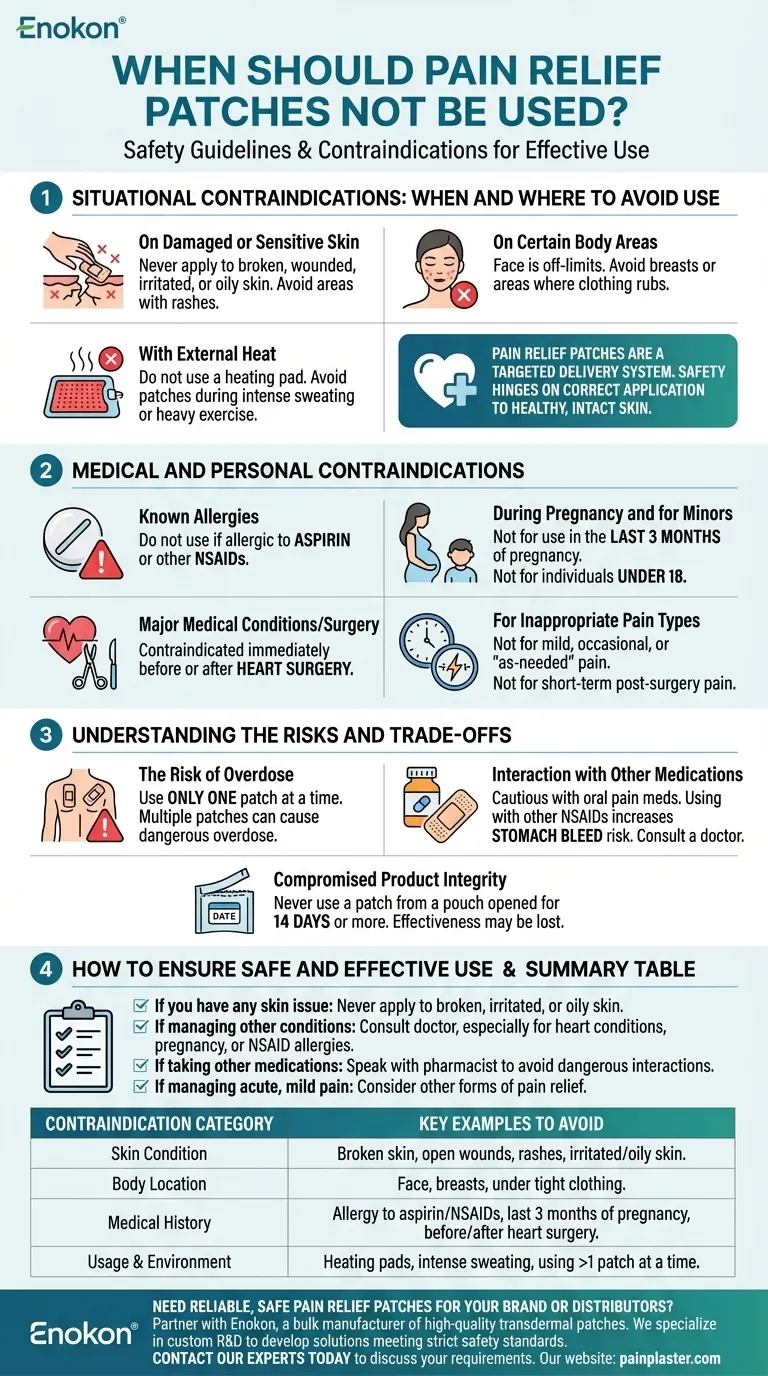
Under no circumstances should a pain relief patch be used on your face, on open wounds, or on rashes and damaged skin. It is also critical to avoid use if you have an allergy to aspirin or other NSAIDs, are in the last three months of pregnancy, or are right before or after heart surgery.
Pain relief patches are a targeted delivery system, and their safety hinges on correct application to healthy, intact skin while avoiding specific medical conditions, external heat sources, and interactions with other medications.

Situational Contraindications: When and Where to Avoid Use
A patch works by delivering medication through the skin. The condition and location of that skin are therefore paramount for both safety and effectiveness.
On Damaged or Sensitive Skin
Never apply a patch to skin that is broken, wounded, irritated, or oily. The medication is not designed to be absorbed through compromised skin, which can lead to adverse reactions or unpredictable dosing.
This restriction also includes areas with rashes. Applying a patch over a rash can worsen the skin condition and interfere with proper medication delivery.
On Certain Body Areas
The face is always an off-limits area for pain patches. Additionally, you should avoid placing patches on the breasts or on areas where clothing might rub it off, such as directly on your waistline.
With External Heat
Do not use a heating pad over a pain relief patch. External heat can accelerate the rate at which the medication is absorbed, potentially leading to a dangerous overdose.
Similarly, avoid using a patch when you will be sweating heavily, such as during intense exercise or in a very hot environment, as this can affect both adhesion and absorption rates.
Medical and Personal Contraindications
Your personal health history and specific medical circumstances play a critical role in determining if a pain patch is a safe option for you.
Known Allergies
If you have a known allergy to aspirin or other NSAIDs (nonsteroidal anti-inflammatory drugs), you must not use pain relief patches containing these ingredients.
Major Medical Conditions or Surgery
Pain patches are contraindicated immediately before or after heart surgery. The specific reasons involve potential effects on bleeding and cardiovascular function.
During Pregnancy and for Minors
The patch should not be used by anyone during the last 3 months of pregnancy. Furthermore, these products are not intended for use by individuals under 18 years of age.
For Inappropriate Pain Types
Some patches, like the buprenorphine patch, are designed for chronic, persistent pain. They should not be used for mild, occasional, or "as-needed" pain, nor for short-term pain following a standard surgery.
Understanding the Risks and Trade-offs
Using a pain patch incorrectly can introduce significant risks, from skin irritation to systemic side effects. Understanding these limitations is key to safe use.
The Risk of Overdose
You must only use one patch at a time. Applying multiple patches simultaneously can deliver a dangerously high dose of medication into your system.
Interaction with Other Medications
Be extremely cautious if you are taking oral pain medications. Using a patch while also taking other NSAIDs increases the risk of side effects like a stomach bleed, even though the chance is small. Always consult a doctor or pharmacist first.
Compromised Product Integrity
Never use a patch from a pouch that has been open for 14 days or more. Over time, the patch can lose its effectiveness and the integrity of the medication may be compromised.
How to Ensure Safe and Effective Use
Your primary goal is to get relief without introducing unnecessary risk. The right choice depends entirely on following safety guidelines and understanding your own health profile.
- If you have any skin issue: Never apply a patch to skin that is broken, irritated, oily, or covered in a rash.
- If you are managing other health conditions: Consult your doctor before use, especially if you have heart conditions, are pregnant, or have NSAID allergies.
- If you are taking other medications: Always speak with a pharmacist or doctor to avoid dangerous drug interactions, particularly with other pain relievers.
- If you are managing acute, mild, or short-term pain: A pain patch may not be the appropriate treatment; consider other forms of pain relief.
Ultimately, using a pain relief patch safely means respecting it as a serious medication and adhering strictly to its guidelines.
Summary Table:
| Contraindication Category | Key Examples to Avoid |
|---|---|
| Skin Condition | Broken skin, open wounds, rashes, irritated or oily skin. |
| Body Location | Face, breasts, areas under tight clothing. |
| Medical History | Allergy to aspirin/NSAIDs, last 3 months of pregnancy, immediately before/after heart surgery. |
| Usage & Environment | Use with heating pads, intense sweating, using more than one patch at a time. |
Need reliable, safe pain relief patches for your brand or distributors? Partner with Enokon, a bulk manufacturer of high-quality transdermal patches and pain plasters. We specialize in custom R&D to develop solutions that meet strict safety and efficacy standards for healthcare and pharmaceutical clients. Contact our experts today to discuss your specific requirements and benefit from our technical expertise.
Visual Guide
Related Products
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Icy Hot Menthol Medicine Pain Relief Patch
- Menthol Gel Pain Relief Patch
- Lidocaine Hydrogel Pain Relief Patch for Pain Relief
- Heat Relief Capsicum Patch for Lower Back Pain Relief
People Also Ask
- How do pain relief patches work? A Guide to Targeted, Long-Lasting Pain Relief
- What are pain relief patches and how are they used? A Guide to Safe, Targeted Relief
- How does the Deep Heat Back Patch work? A Drug-Free Solution for Targeted Pain Relief
- How do Deep Heat Pain Relief Patches provide pain relief? Discover the Drug-Free Mechanism
- How often should pain relief patches be used? Get the Right Schedule for Targeted Relief


















