The Lidoderm patch, a topical analgesic patch, received FDA approval in 1999. This approval marked its introduction as a medical device for pain relief, leveraging lidocaine to provide localized treatment. The patch's design and efficacy met the regulatory standards required for such products, ensuring its availability for patients needing non-invasive pain management solutions.
Key Points Explained:
-
FDA Approval Year:
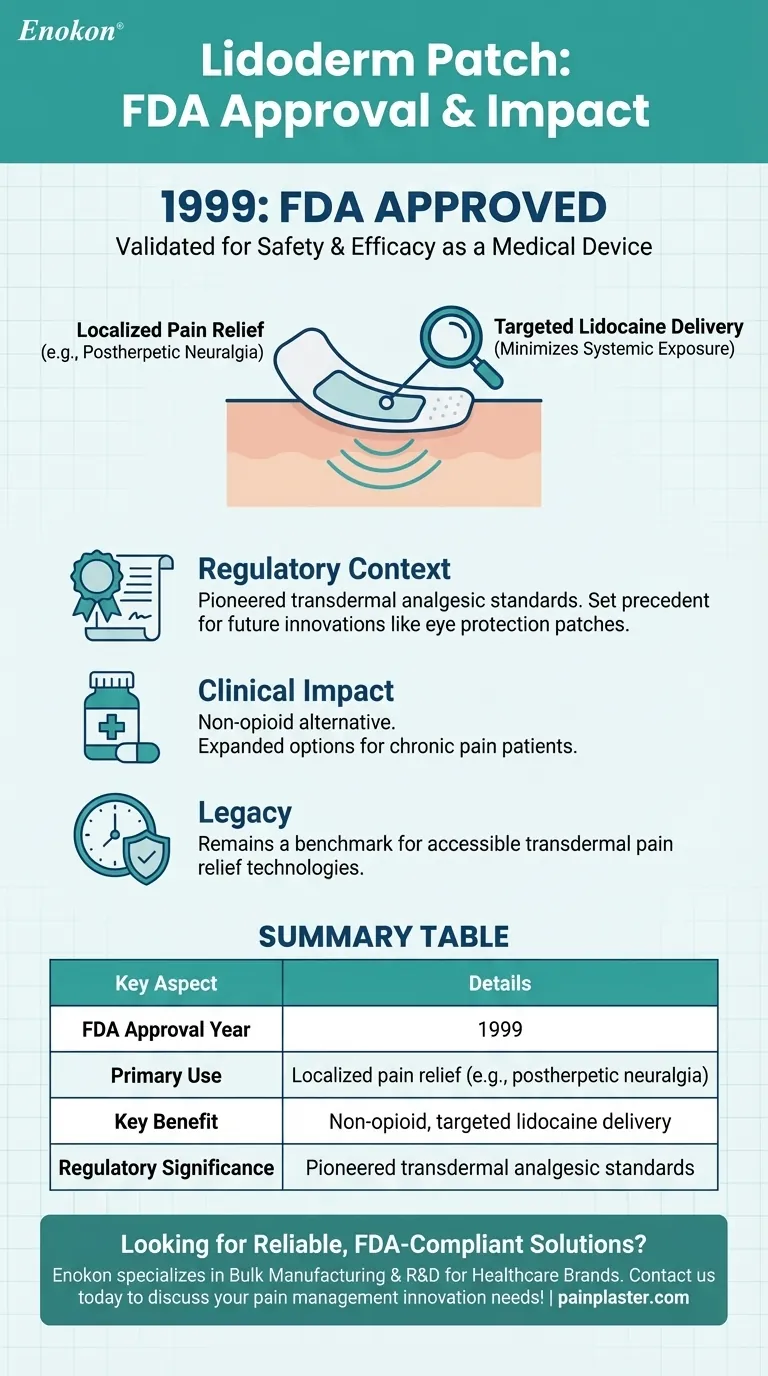
- The Lidoderm patch was approved by the U.S. Food and Drug Administration (FDA) in 1999. This milestone validated its safety and efficacy for clinical use.
- The approval process involved rigorous testing to ensure the patch met standards for delivering lidocaine effectively without systemic side effects.
-
Purpose and Design:
- The patch is designed to provide localized pain relief, particularly for conditions like postherpetic neuralgia (shingles-related pain).
- Its adhesive backing allows for targeted delivery of lidocaine, minimizing exposure to other body areas.
-
Regulatory Context:
- FDA approval in 1999 placed the Lidoderm patch among early topical analgesic innovations.
- It set a precedent for similar products, including the eye protection patch, which followed later for different therapeutic applications.
-
Clinical Impact:
- The patch offered a non-opioid alternative for pain management, aligning with growing emphasis on reducing systemic drug use.
- Its approval expanded options for patients with chronic pain, particularly those sensitive to oral medications.
The Lidoderm patch remains a benchmark for transdermal pain relief, reflecting the FDA’s role in advancing accessible healthcare technologies.
Summary Table:
| Key Aspect | Details |
|---|---|
| FDA Approval Year | 1999 |
| Primary Use | Localized pain relief (e.g., postherpetic neuralgia) |
| Key Benefit | Non-opioid, targeted lidocaine delivery with minimal systemic exposure |
| Regulatory Significance | Pioneered transdermal analgesic standards |
Looking for reliable, FDA-compliant transdermal pain relief solutions? Enokon specializes in bulk manufacturing of high-quality analgesic patches and custom R&D for healthcare brands. Contact us today to discuss your needs and leverage our expertise in pain management innovation!
Visual Guide
Related Products
- Herbal Eye Protection Patch Eye Patch
- Lidocaine Hydrogel Pain Relief Patch for Pain Relief
- Hydra Gel Health Care Eye Patch
- Icy Hot Menthol Medicine Pain Relief Patch
- Asthma Cough and Pain Relief Patch for Adults and Kids
People Also Ask
- How quickly can you see results from using under eye patches? Instant Brightening & Long-Term Benefits
- How do eye patches enhance the effectiveness of eye creams? Boost Your Eye Care Routine
- How can using eye patches contribute to a self-care skincare routine? Boost Hydration & Relaxation
- What are the steps for applying under-eye patches? Boost Your Eye Care Routine
- What are the main benefits of using eye patches in a skincare routine? Revitalize Your Under-Eye Area
















