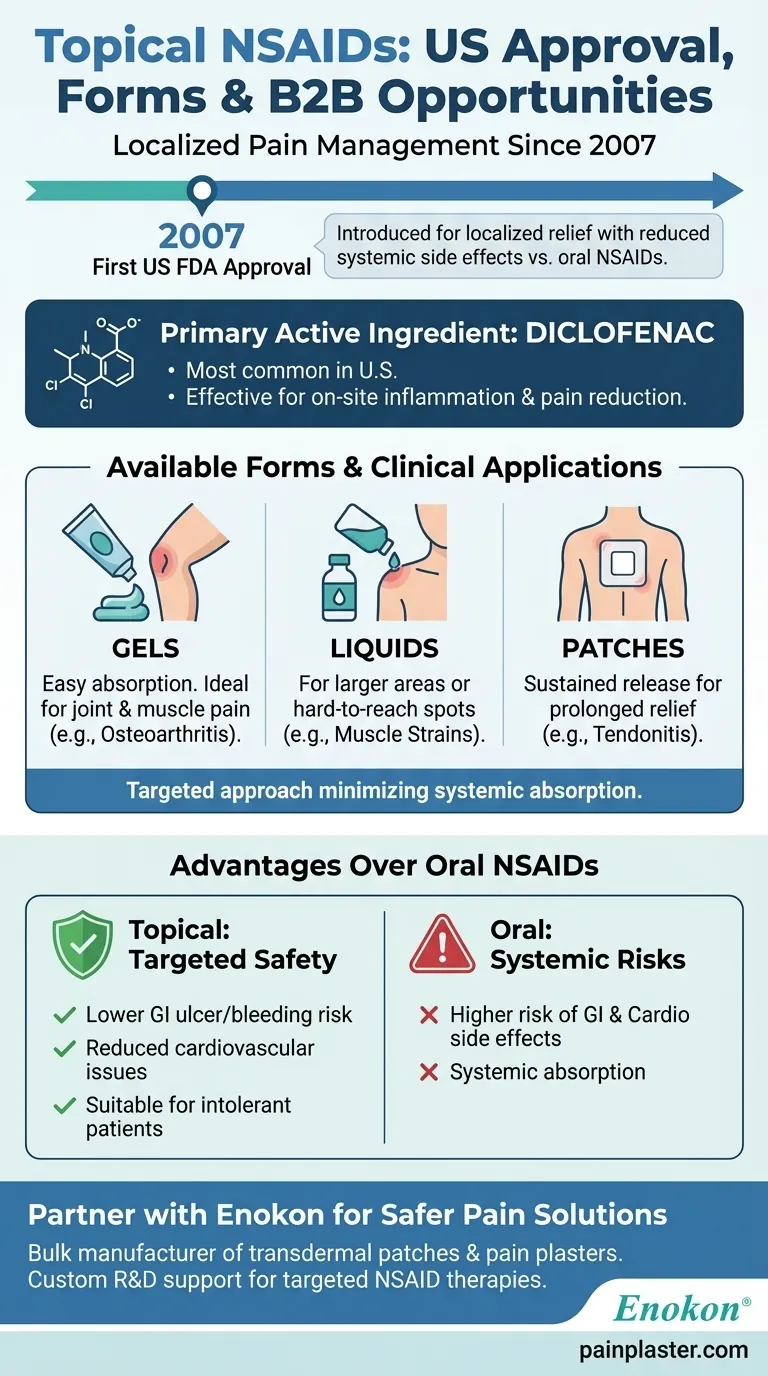
Topical NSAIDs (Non-Steroidal Anti-Inflammatory Drugs) were introduced in the United States in 2007, marking a significant milestone in pain management. These medications provide localized relief with fewer systemic side effects compared to oral NSAIDs. The primary form available is diclofenac, which is offered in various formulations like gels, liquids, and patches to cater to different patient needs and preferences. This development has expanded treatment options for conditions like osteoarthritis and muscle pain, offering patients a safer and more targeted approach to pain relief.
Key Points Explained:
-
First Approval in the U.S. (2007)
- Topical NSAIDs were first approved by the FDA in 2007, providing a new avenue for pain management.
- This approval addressed the need for localized treatment options with reduced gastrointestinal and cardiovascular risks compared to oral NSAIDs.
-
Primary Active Ingredient: Diclofenac
- Diclofenac is the most commonly used topical NSAID in the U.S.
- It is favored for its efficacy in reducing inflammation and pain at the application site.
-
Available Forms
- Gels: Easy to apply and absorb, ideal for joint or muscle pain.
- Liquids: Often used for larger surface areas or hard-to-reach spots.
- Patches: Provide sustained release of medication, convenient for prolonged pain relief.
-
Clinical Applications
- Primarily used for osteoarthritis, tendonitis, and muscle strains.
- Offers a targeted approach, minimizing systemic absorption and side effects.
-
Advantages Over Oral NSAIDs
- Lower risk of gastrointestinal ulcers, bleeding, and cardiovascular issues.
- Suitable for patients who cannot tolerate oral NSAIDs or have comorbidities.
The introduction of topical NSAIDs has revolutionized pain management, providing patients with effective, localized relief while mitigating the risks associated with systemic treatments. Have you considered how these formulations might benefit specific patient populations, such as the elderly or those with chronic conditions?
Summary Table:
| Key Aspect | Details |
|---|---|
| First FDA Approval | 2007, offering localized pain relief with fewer systemic risks. |
| Primary Ingredient | Diclofenac (effective for inflammation and site-specific pain). |
| Available Forms | Gels (easy absorption), liquids (large areas), patches (sustained release). |
| Clinical Uses | Osteoarthritis, tendonitis, muscle strains. |
| Advantages Over Oral NSAIDs | Lower risk of ulcers, bleeding, and cardiovascular issues. |
Upgrade your pain management solutions with Enokon’s expertise!
As a bulk manufacturer of transdermal patches and pain plasters, we help healthcare distributors and pharma brands deliver safer, targeted NSAID therapies. Benefit from our custom R&D support to develop formulations tailored to your patients' needs—contact us today to discuss partnerships!
Visual Guide
Related Products
- Icy Hot Menthol Medicine Pain Relief Patch
- Hydra Gel Health Care Eye Patch
- Mugwort Wormwood Pain Relief Patch for Neck Pain
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Prostate Pain Kidney Health Care Patch for Men
People Also Ask
- Are cooling patches reusable? Understanding Single-Use Cooling Solutions
- How does menthol work in the Reliever Patch? Dual-Action Pain Relief Explained
- What are common side effects of menthol patch? Key Risks & Safety Tips
- How does menthol function as a topical analgesic? The Science Behind Cooling Pain Relief
- What are the pharmacokinetics of topical menthol application? Rapid Absorption & Short-Term Relief Explained
















