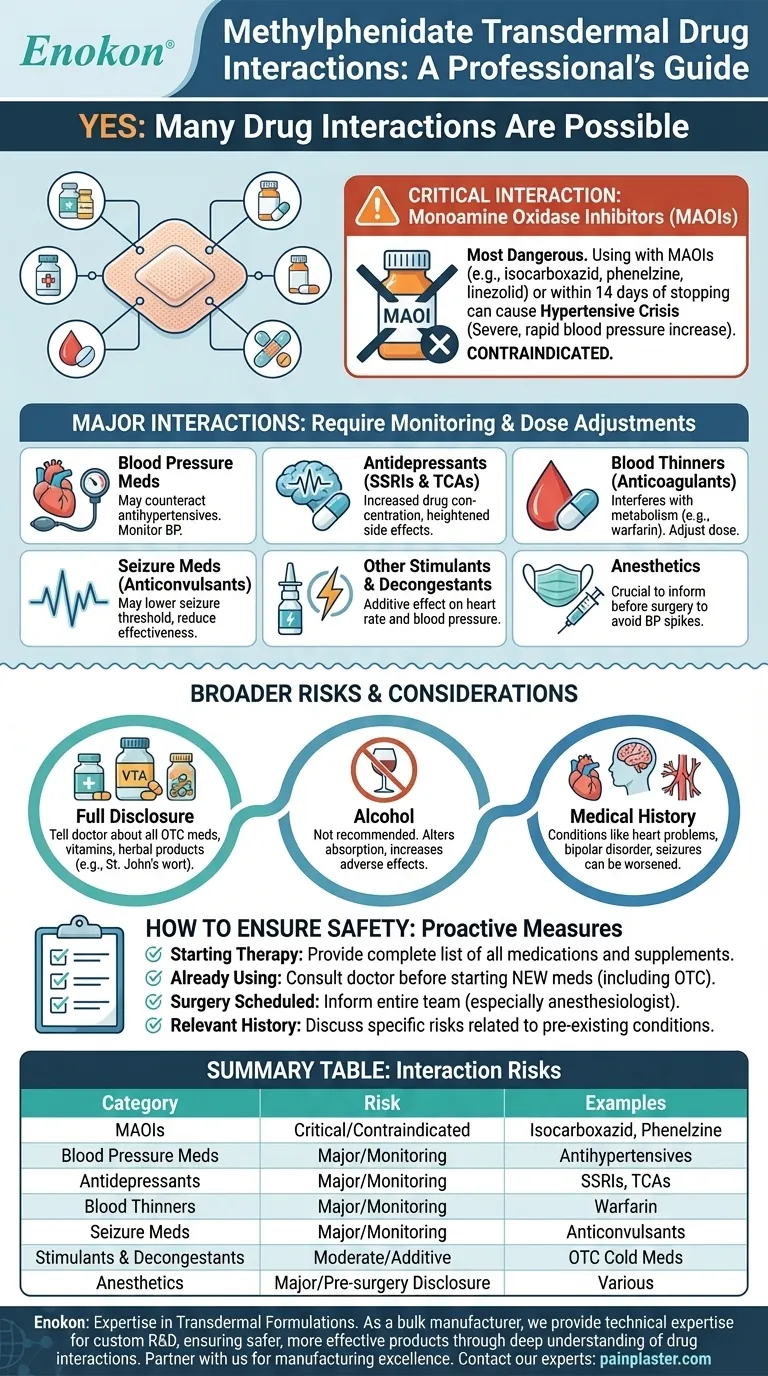
Yes, several categories of drugs can interact with methylphenidate transdermal. The most critical interactions involve a class of antidepressants known as MAO inhibitors. However, potential interactions also exist with common medications for blood pressure, seizures, and depression, as well as some over-the-counter products.
The central takeaway is that while many drug interactions are possible, the most severe risk comes from combining methylphenidate with Monoamine Oxidase Inhibitors (MAOIs). For all other medications, the key to safety is ensuring your doctor has a complete and current list of everything you take, including supplements and over-the-counter drugs.
Critical Interactions to Avoid
Certain drug combinations pose a significant risk and are generally contraindicated.
Monoamine Oxidase Inhibitors (MAOIs)
This is the most dangerous interaction. Using methylphenidate while taking an MAOI, or within 14 days of stopping one, can lead to a hypertensive crisis—a severe, rapid increase in blood pressure that can be life-threatening.
MAOIs include drugs like isocarboxazid, phenelzine, tranylcypromine, and the antibiotic linezolid.
Major Interactions Requiring Monitoring
Other medications may not be strictly forbidden but require careful monitoring or dose adjustments by your doctor when used with the methylphenidate patch.
Blood Pressure Medications
Methylphenidate is a stimulant and can increase blood pressure and heart rate. This can counteract the effects of medications designed to lower blood pressure, making them less effective.
Antidepressants (SSRIs & Tricyclics)
Combining methylphenidate with certain antidepressants, such as Selective Serotonin Reuptake Inhibitors (SSRIs) or Tricyclic Antidepressants (TCAs), may increase the concentration of these drugs in the blood, potentially heightening their side effects.
Blood Thinners (Anticoagulants)
Methylphenidate can potentially interfere with the metabolism of blood thinners like warfarin. This interaction may require your doctor to adjust the dose of your anticoagulant to maintain its effectiveness and safety.
Seizure Medications (Anticonvulsants)
Methylphenidate may lower the seizure threshold in some individuals. This is a particular concern for patients taking anticonvulsant medications, as it could potentially reduce their effectiveness.
Other Stimulants and Decongestants
Using other stimulant medications alongside methylphenidate can have an additive effect. This includes prescription stimulants and many over-the-counter decongestants, which can increase heart rate and blood pressure.
Anesthetics
It is crucial to inform your surgeon and anesthesiologist that you use a methylphenidate patch before any planned surgery. The combination with certain anesthetics can lead to sudden increases in blood pressure during a procedure.
Understanding the Broader Risks
Effective management goes beyond just drug-to-drug interactions. Your overall health profile plays a significant role in the safety of your treatment.
The Importance of Full Disclosure
The risk of interaction isn't limited to prescription drugs. You must inform your doctor about all over-the-counter medicines, vitamins, and herbal supplements (like St. John's wort) you are taking.
The Role of Alcohol
Using alcohol with methylphenidate is not recommended. It can alter the way the drug is absorbed and may increase the risk of adverse side effects.
Pre-existing Medical Conditions
Certain health conditions can be worsened by methylphenidate. It is vital your doctor knows if you have a history of heart conditions, high blood pressure, bipolar disorder, psychosis, seizures, or circulation problems like Raynaud's phenomenon.
How to Ensure Your Safety
Proactive communication with your healthcare provider is the most effective strategy for managing potential interactions.
- If you are starting methylphenidate transdermal: Provide your doctor with a complete list of every medication, supplement, and herbal product you currently take.
- If you are already using the patch: Always consult your doctor before starting any new medication, especially over-the-counter drugs like cold and allergy decongestants.
- If you are scheduled for surgery: Ensure your entire surgical team, particularly the anesthesiologist, is aware that you use a methylphenidate patch.
- If you have a relevant medical history: Discuss the specific risks associated with your health conditions, such as heart problems or high blood pressure, before beginning treatment.
Transparent partnership with your healthcare team is the cornerstone of safe and effective treatment.
Summary Table:
| Category of Medication | Potential Interaction Risk | Key Examples |
|---|---|---|
| Monoamine Oxidase Inhibitors (MAOIs) | Critical / Contraindicated | Isocarboxazid, Phenelzine, Linezolid |
| Blood Pressure Medications | Major / Requires Monitoring | Various antihypertensives |
| Antidepressants (SSRIs, TCAs) | Major / Requires Monitoring | Sertraline, Amitriptyline |
| Blood Thinners (Anticoagulants) | Major / Requires Monitoring | Warfarin |
| Seizure Medications (Anticonvulsants) | Major / Requires Monitoring | Various anticonvulsants |
| Other Stimulants & Decongestants | Moderate / Additive Effect | OTC cold medicines |
| Anesthetics | Major / Pre-surgery Disclosure | Various anesthetics |
Ensure the safety and efficacy of your transdermal drug formulations.
As a bulk manufacturer of reliable transdermal patches and pain plasters, Enokon provides healthcare and pharma distributors and brands with the technical expertise needed for custom R&D and development. Our deep understanding of drug interactions and formulation science helps you deliver safer, more effective products to market.
Partner with us to leverage our manufacturing excellence for your next project.
Contact our experts today to discuss your needs
Visual Guide
Related Products
- Icy Hot Menthol Medicine Pain Relief Patch
- Mugwort Wormwood Pain Relief Patch for Neck Pain
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Capsaicin Chili Medicated Pain Relief Patches
- Menthol Gel Pain Relief Patch
People Also Ask
- Are cooling patches reusable? Understanding Single-Use Cooling Solutions
- How should a menthol patch be applied? Follow These Steps for Safe & Effective Pain Relief
- How does menthol in the patch work to relieve pain? Discover the Science Behind Fast-Acting Relief
- What are the pharmacokinetics of topical menthol application? Rapid Absorption & Short-Term Relief Explained
- Can cooling patches be used on newborns? Safe Fever Relief for Infants













