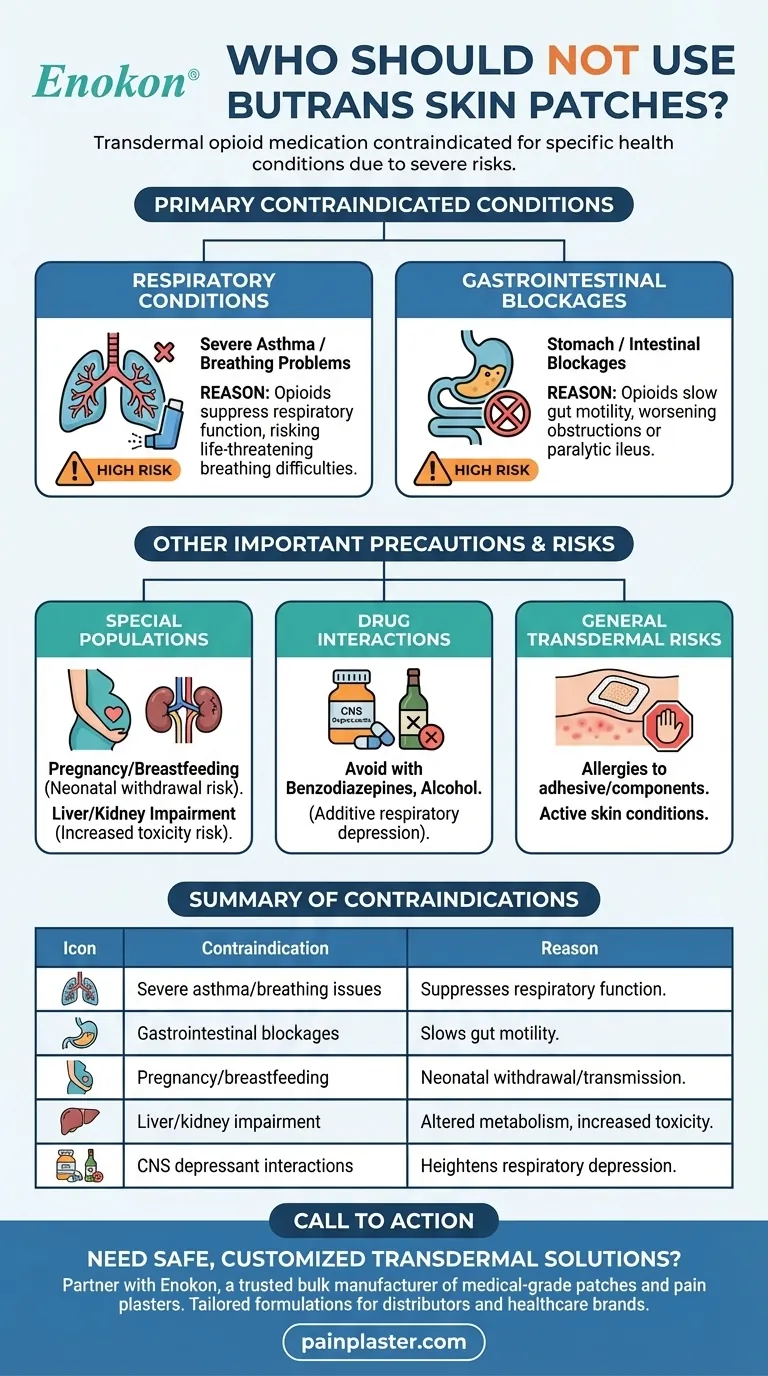
Butrans skin patches, a transdermal opioid medication, are contraindicated for individuals with specific health conditions due to increased risks of severe complications. Primarily, those with severe asthma or breathing problems should avoid Butrans, as opioids can suppress respiratory function, potentially leading to life-threatening breathing difficulties. Similarly, individuals with gastrointestinal blockages should not use Butrans, as opioids can exacerbate intestinal motility issues. Additionally, while not directly related to Butrans, insights from HRT patches suggest that individuals with certain allergies, cancer histories, or active conditions like untreated hyperplasia may also need caution with transdermal medications, though these are more relevant to hormonal therapies than opioids.

Key Points Explained:
-
Respiratory Conditions
- Severe Asthma/Breathing Problems: Butrans contains buprenorphine, an opioid that can depress the central nervous system and reduce respiratory drive. This is particularly dangerous for those with pre-existing compromised lung function, such as severe asthma or chronic obstructive pulmonary disease (COPD).
- Consideration: Have you discussed alternative pain management options with your healthcare provider if you have a respiratory condition?
-
Gastrointestinal Blockages
- Stomach/Intestinal Blockages: Opioids like buprenorphine slow gut motility, which can worsen obstructions or paralytic ileus. This is critical for patients with conditions like Crohn’s disease or postoperative adhesions.
-
General Contraindications from HRT Insights
- While not specific to Butrans, HRT patch warnings highlight broader transdermal risks:
- Allergies: Hypersensitivity to adhesive components or active ingredients.
- Cancer History: Hormone-sensitive cancers (e.g., breast) may recur with estrogen/progestogen, but this is less relevant to opioids.
- Active Conditions: Untreated endometrial hyperplasia or blood clotting disorders may require caution with any transdermal therapy due to systemic absorption.
- While not specific to Butrans, HRT patch warnings highlight broader transdermal risks:
-
Special Populations
- Pregnancy/Breastfeeding: Opioids can cause neonatal withdrawal or pass into breast milk.
- Liver/Kidney Impairment: Buprenorphine metabolism may be affected, increasing toxicity risks.
-
Drug Interactions
- Butrans should not be used with other CNS depressants (e.g., benzodiazepines, alcohol) due to additive respiratory depression.
Final Thought: Transdermal patches offer convenience but require careful screening for underlying health issues—whether for pain relief or hormone therapy. Always review your medical history with a clinician before starting any patch-based treatment.
Summary Table:
| Contraindication | Reason |
|---|---|
| Severe asthma/breathing issues | Opioids suppress respiratory function, risking life-threatening complications. |
| Gastrointestinal blockages | Slowed gut motility can worsen obstructions or paralytic ileus. |
| Pregnancy/breastfeeding | Risk of neonatal withdrawal or transmission through breast milk. |
| Liver/kidney impairment | Altered metabolism may increase toxicity. |
| CNS depressant interactions | Combined use (e.g., benzodiazepines, alcohol) heightens respiratory depression. |
Need safe, customized transdermal solutions? Partner with Enokon, a trusted bulk manufacturer of medical-grade patches and pain plasters. Our expertise in transdermal R&D ensures tailored formulations for distributors and healthcare brands. Contact us to discuss compliant, high-quality alternatives for your patients.
Visual Guide
Related Products
- Heat Relief Capsicum Patch for Lower Back Pain Relief
- Lidocaine Hydrogel Pain Relief Patch for Pain Relief
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Capsaicin Chili Medicated Pain Relief Patches
- Heating Pain Relief Patches for Menstrual Cramps
People Also Ask
- How does the Deep Heat Back Patch work? A Drug-Free Solution for Targeted Pain Relief
- What types of pain can the Deep Heat Pain Relief Back Patch be used for? Targeted Relief for Muscles & Joints
- What precautions should be taken when using the Capsicum HOT PATCH Adhesive Patch? Essential Safety Tips
- How should the Capsicum HOT PATCH Adhesive Patch be stored? Best Practices for Safety & Effectiveness
- What should be done if a dose of the Capsicum HOT PATCH Adhesive Patch is missed? Follow These Steps for Safe Use

















