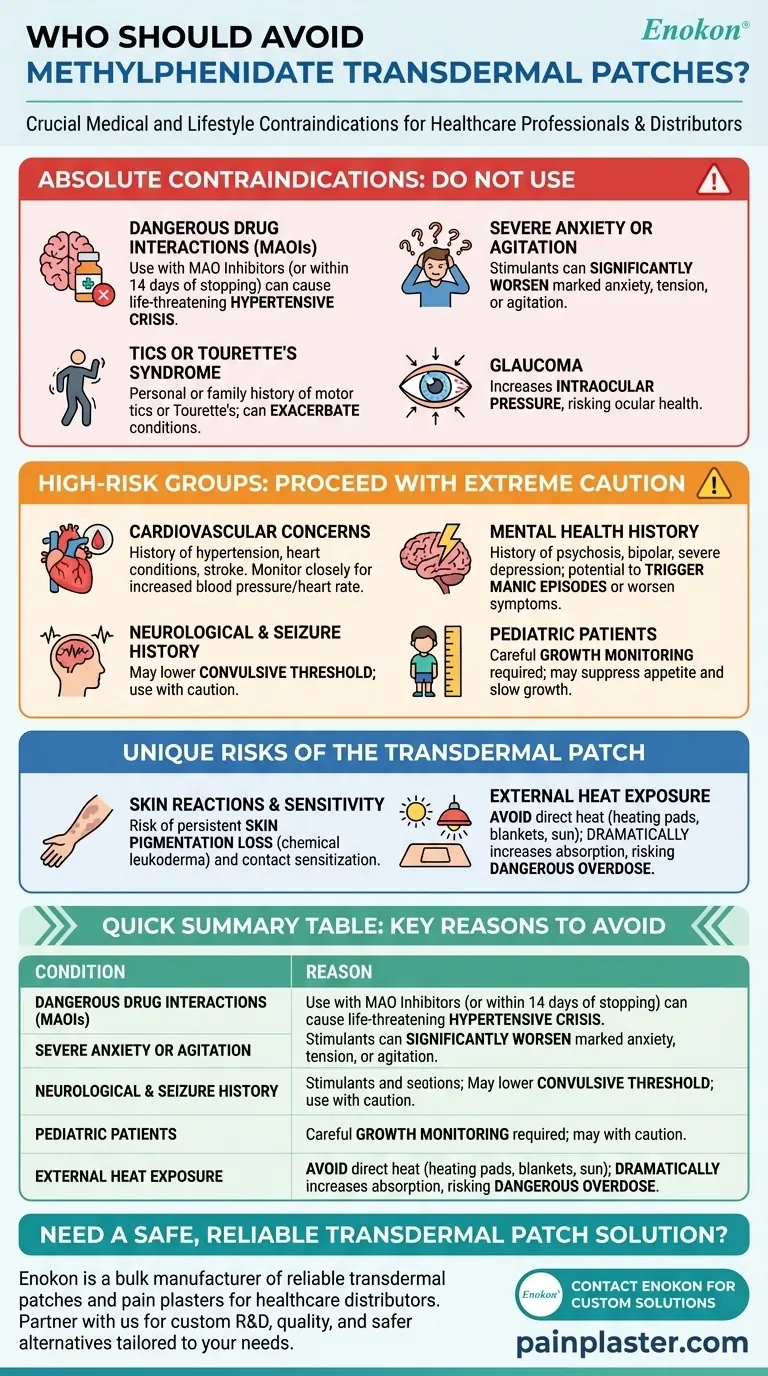
Methylphenidate transdermal is not suitable for everyone, and several key conditions and medication interactions strictly prohibit its use. Individuals with a known allergy to methylphenidate, those with severe anxiety or glaucoma, a personal or family history of tics or Tourette's syndrome, or anyone who has taken an MAO inhibitor within the past 14 days should not use this medication.
The decision to avoid methylphenidate transdermal goes beyond the drug itself; it involves considering both the psychiatric and cardiovascular risks common to stimulants, as well as unique skin-related and heat-exposure risks specific to the patch delivery system.

Absolute Contraindications: When Not to Use This Medication
Certain conditions and drug combinations create an unacceptable level of risk, making the use of a methylphenidate patch unsafe. These are known as absolute contraindications.
Dangerous Drug Interactions (MAO Inhibitors)
A critical contraindication is the use of a monoamine oxidase inhibitor (MAOI), a class of medication often used for depression.
Using methylphenidate while taking an MAOI, or within 14 days of stopping one, can lead to a hypertensive crisis—a sudden and severe increase in blood pressure that can be life-threatening.
Pre-existing Psychiatric Conditions
This medication is not for individuals with marked anxiety, tension, or agitation.
As a central nervous system stimulant, methylphenidate can significantly worsen these symptoms.
Neurological and Ocular Health
The patch is contraindicated for individuals with motor tics or a personal or family history of Tourette's syndrome. Stimulants can exacerbate these conditions.
Additionally, people with glaucoma should not use this medication, as it can increase pressure within the eye.
Known Allergies and Skin Reactions
If you have a known hypersensitivity or allergy to methylphenidate or any other component of the patch, you must not use it.
A history of a skin reaction to a previous methylphenidate patch, known as contact sensitization, is also a reason to avoid it.
High-Risk Groups: Proceed with Extreme Caution
Beyond absolute contraindications, several medical histories require a careful risk-benefit analysis with a healthcare provider. The presence of these conditions may lead a doctor to recommend against the patch.
Cardiovascular Concerns
Patients with a history of hypertension, heart conditions, blood vessel problems (like Raynaud phenomenon), or stroke must be monitored closely.
Methylphenidate can increase blood pressure and heart rate, which can be dangerous for those with underlying cardiovascular issues.
Mental Health History
For individuals with a history of psychosis, bipolar disorder, or severe depression, stimulants can potentially trigger manic episodes or worsen psychotic symptoms.
Neurological and Seizure History
Patients with a history of seizures should use this medication with caution. Methylphenidate may lower the convulsive threshold, potentially increasing the risk of having a seizure.
Pediatric Patients
For children and adolescents, the use of methylphenidate requires careful monitoring of growth (height and weight), as stimulants can sometimes suppress appetite and slow growth.
Understanding the Trade-offs of the Transdermal Patch
The patch delivery system introduces unique risks that are not present with oral formulations of the same medication. Understanding these is critical for safe use.
The Risk of Skin Reactions
Applying the patch can lead to skin issues at the application site. This includes persistent loss of skin pigmentation (chemical leukoderma), which may be permanent.
The Danger of External Heat
You must avoid exposing the application site to direct external heat sources. This includes heating pads, electric blankets, hair dryers, or prolonged sun exposure.
Heat dramatically increases the rate at which the medication is absorbed through the skin, which can lead to a sudden, dangerous overdose.
Impaired Judgment and Motor Skills
The medication may cause dizziness, drowsiness, or blurred vision.
Until you know how the patch affects you, you must avoid driving, operating heavy machinery, or performing any other hazardous activity.
Making an Informed Decision with Your Doctor
A comprehensive evaluation by a healthcare professional is essential before starting this medication. Be prepared to discuss your entire medical history.
- If you have a history of skin sensitivity or vitiligo: The transdermal patch carries unique risks of sensitization and pigmentation loss that oral medications do not.
- If you have a heart condition or high blood pressure: Any stimulant, including this one, requires careful cardiovascular monitoring and may be deemed too risky for your specific condition.
- If you take other medications, especially for mental health: You must disclose all medications to your doctor due to the high potential for serious interactions, particularly with MAOIs.
- If your lifestyle involves frequent exposure to heat: The patch poses a significant risk of overdose due to heat-accelerated absorption and should likely be avoided.
Ultimately, a thorough discussion of your complete health history with your healthcare provider is the only way to determine if this specific medication is a safe choice for you.
Summary Table:
| Who Should Avoid? | Primary Reason(s) |
|---|---|
| Taking MAOIs (or within 14 days) | Risk of life-threatening hypertensive crisis |
| Known Methylphenidate Allergy | Risk of severe hypersensitivity reaction |
| History of Severe Anxiety/Agitation | Stimulant can significantly worsen symptoms |
| Personal/Family History of Tics/Tourette's | Can exacerbate neurological conditions |
| Glaucoma | Can increase intraocular pressure |
| Serious Heart Conditions | Can increase blood pressure and heart rate |
| History of Skin Sensitization to Patch | Risk of contact sensitization and pigmentation loss |
| Frequent Exposure to External Heat | Heat can cause dangerous overdose via accelerated absorption |
Need a Safe, Reliable Transdermal Patch Solution?
If your patients require a transdermal delivery system but have sensitivities or specific health considerations, a custom formulation may be the answer. Enokon is a bulk manufacturer of reliable transdermal patches and pain plasters for healthcare and pharmaceutical distributors and brands.
We can help you develop a safer alternative:
- Custom R&D: Our technical expertise allows for the development of patches with different active pharmaceutical ingredients (APIs) or tailored release profiles to mitigate risks.
- Quality & Reliability: We ensure every patch meets stringent quality and safety standards for consistent performance.
- Expert Collaboration: Partner with us to create a transdermal solution that aligns with your patients' unique health profiles and safety needs.
Let's discuss a custom patch solution for your brand. Contact our experts today to explore how we can support your product development goals.
Visual Guide
Related Products
- Icy Hot Menthol Medicine Pain Relief Patch
- Mugwort Wormwood Pain Relief Patch for Neck Pain
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Capsaicin Chili Medicated Pain Relief Patches
- Menthol Gel Pain Relief Patch
People Also Ask
- How does menthol function as a topical analgesic? The Science Behind Cooling Pain Relief
- How does menthol in the patch work to relieve pain? Discover the Science Behind Fast-Acting Relief
- Is menthol topical safe during pregnancy and breastfeeding? Key Safety Insights
- Can cooling patches be used on newborns? Safe Fever Relief for Infants
- Are cooling patches reusable? Understanding Single-Use Cooling Solutions













