The rivastigmine transdermal patch is not suitable for everyone. It should never be used if you have a known allergy to rivastigmine or similar carbamate-derived medicines. Crucially, you must also avoid it if you have ever had a severe skin reaction to a previous rivastigmine patch that spread beyond the application site.
While a history of specific allergies or severe skin reactions are absolute reasons to avoid the rivastigmine patch, several other health conditions involving the heart, liver, or stomach require a thorough discussion with your doctor to weigh the risks.
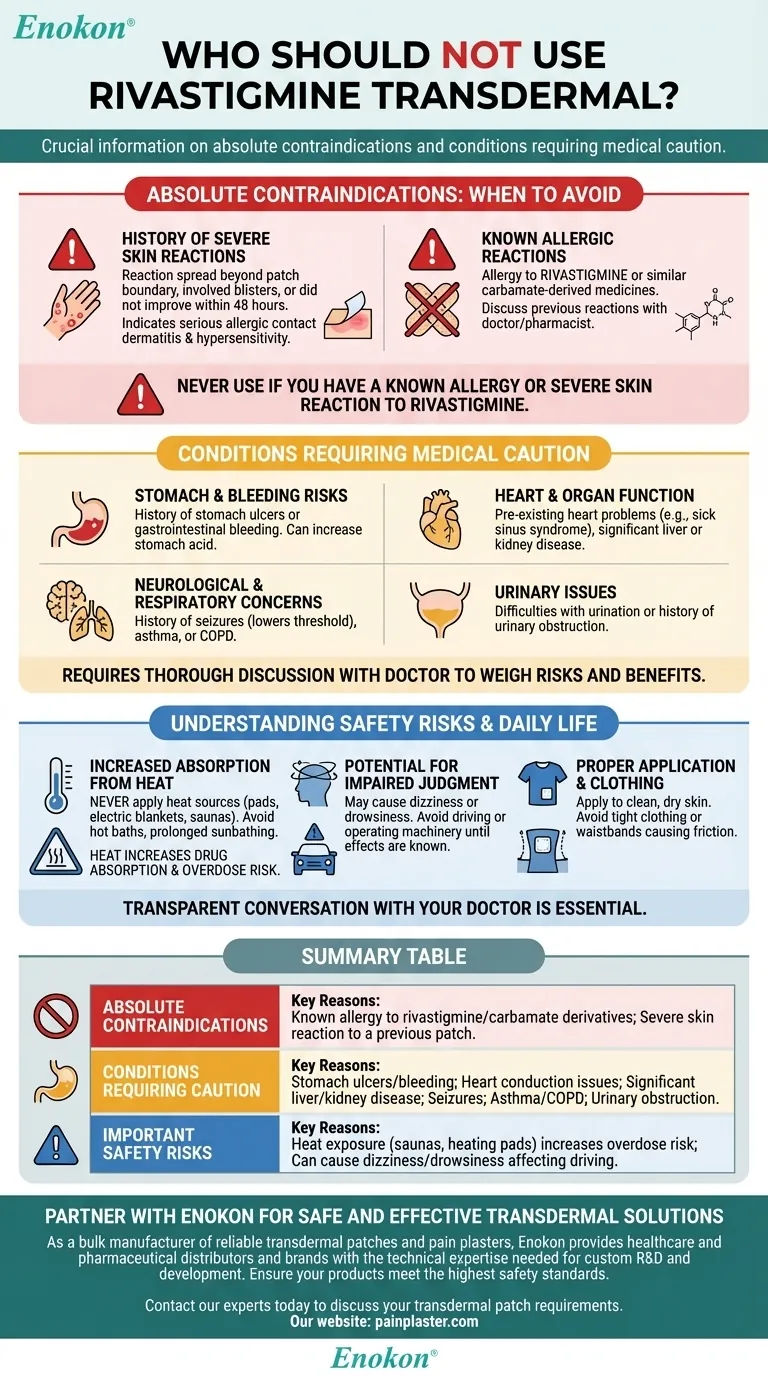
Absolute Contraindications: When to Avoid Rivastigmine Patch
Certain conditions make using the rivastigmine patch unsafe. These are not matters of caution but are clear contraindications for its use.
History of Severe Skin Reactions
If a previous rivastigmine patch caused a skin reaction that spread beyond the patch's boundary, involved blisters, or did not improve within 48 hours of removing the patch, this is considered a serious allergic contact dermatitis.
This indicates a hypersensitivity, and using the patch again could lead to a more severe, systemic reaction.
Known Allergic Reactions
You must not use this medication if you have a known allergy to rivastigmine itself.
This also applies to allergies to similar medications known as carbamate derivatives. Your doctor or pharmacist can help you identify if any medications you've reacted to in the past fall into this category.
Conditions Requiring Medical Caution
While not absolute contraindications, the following conditions require a careful risk-benefit analysis with your doctor before starting treatment. The presence of these issues may require closer monitoring or an alternative treatment.
Stomach and Bleeding Risks
Inform your doctor if you have a history of stomach ulcers or gastrointestinal bleeding. Rivastigmine can increase stomach acid, potentially worsening these conditions.
Heart and Organ Function
Patients with pre-existing heart problems, such as sick sinus syndrome or conduction issues, need careful evaluation.
Likewise, individuals with significant liver or kidney disease may process the medication differently, increasing the risk of side effects.
Neurological and Respiratory Concerns
A history of seizures should be reported, as this class of medication can potentially lower the seizure threshold.
You must also discuss any respiratory disorders like asthma or chronic obstructive pulmonary disease (COPD), as the medication can have effects on the lungs.
Urinary Issues
Difficulties with urination or a history of urinary obstruction should be discussed with your healthcare provider, as this medication can sometimes affect the urinary system.
Understanding the Trade-offs and Safety Precautions
Beyond specific health conditions, daily activities and external factors can significantly alter the medication's effects and must be managed carefully.
The Risk of Increased Absorption
Never apply external heat sources like heating pads, electric blankets, or saunas to the patch site. Do not take hot baths or sunbathe for long periods.
Heat dramatically increases the rate at which the drug is absorbed into your bloodstream, which can lead to an accidental overdose and serious side effects.
Potential for Impaired Judgment
The medication can cause dizziness or drowsiness, particularly when you first start treatment or increase your dose.
Avoid driving, operating heavy machinery, or performing other hazardous activities until you know how the rivastigmine patch affects you.
Proper Application and Clothing
Ensure the patch is applied correctly to clean, dry, and intact skin. Avoid placing it under tight clothing or waistbands, as constant friction can irritate the skin and affect medication delivery.
Making an Informed Decision with Your Doctor
Use this information to have a productive and detailed conversation with your healthcare provider. Your specific health profile will determine the best course of action.
- If your primary focus is safety due to past allergies: You must report any prior skin reaction to a patch, no matter how minor, before considering this treatment.
- If your primary focus is managing existing health conditions: Your doctor must evaluate your heart, kidney, and liver function to confirm the potential benefits outweigh the risks.
- If your primary focus is understanding daily risks: Be prepared to discuss lifestyle factors, such as driving habits and the use of external heat sources, to create a safe treatment plan.
Ultimately, a transparent conversation with your healthcare provider is the essential step to safely determine if the rivastigmine patch is right for you.
Summary Table:
| Category | Key Reasons to Avoid or Use with Caution |
|---|---|
| Absolute Contraindications | Known allergy to rivastigmine/carbamate derivatives; Severe skin reaction to a previous patch. |
| Conditions Requiring Caution | Stomach ulcers/bleeding; Heart conduction issues; Significant liver/kidney disease; Seizures; Asthma/COPD; Urinary obstruction. |
| Important Safety Risks | Heat exposure (saunas, heating pads) increases overdose risk; Can cause dizziness/drowsiness affecting driving. |
Partner with Enokon for Safe and Effective Transdermal Solutions
As a bulk manufacturer of reliable transdermal patches and pain plasters, Enokon provides healthcare and pharmaceutical distributors and brands with the technical expertise needed for custom R&D and development. Ensure your products meet the highest safety standards.
Contact our experts today to discuss your transdermal patch requirements.
Visual Guide
Related Products
- Herbal Eye Protection Patch Eye Patch
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Heating Pain Relief Patches for Menstrual Cramps
- Menthol Gel Pain Relief Patch
People Also Ask
- What are the steps for properly using eye patches? Maximize Benefits for Your Delicate Eye Area
- Can under eye patches smooth fine lines and wrinkles? Hydrate & Plump for Youthful Skin
- How quickly can you see results from using under eye patches? Instant Brightening & Long-Term Benefits
- How do eye patches enhance the effectiveness of eye creams? Boost Your Eye Care Routine
- What factors should be considered when purchasing eye patches? Essential Guide for Safe & Effective Use














