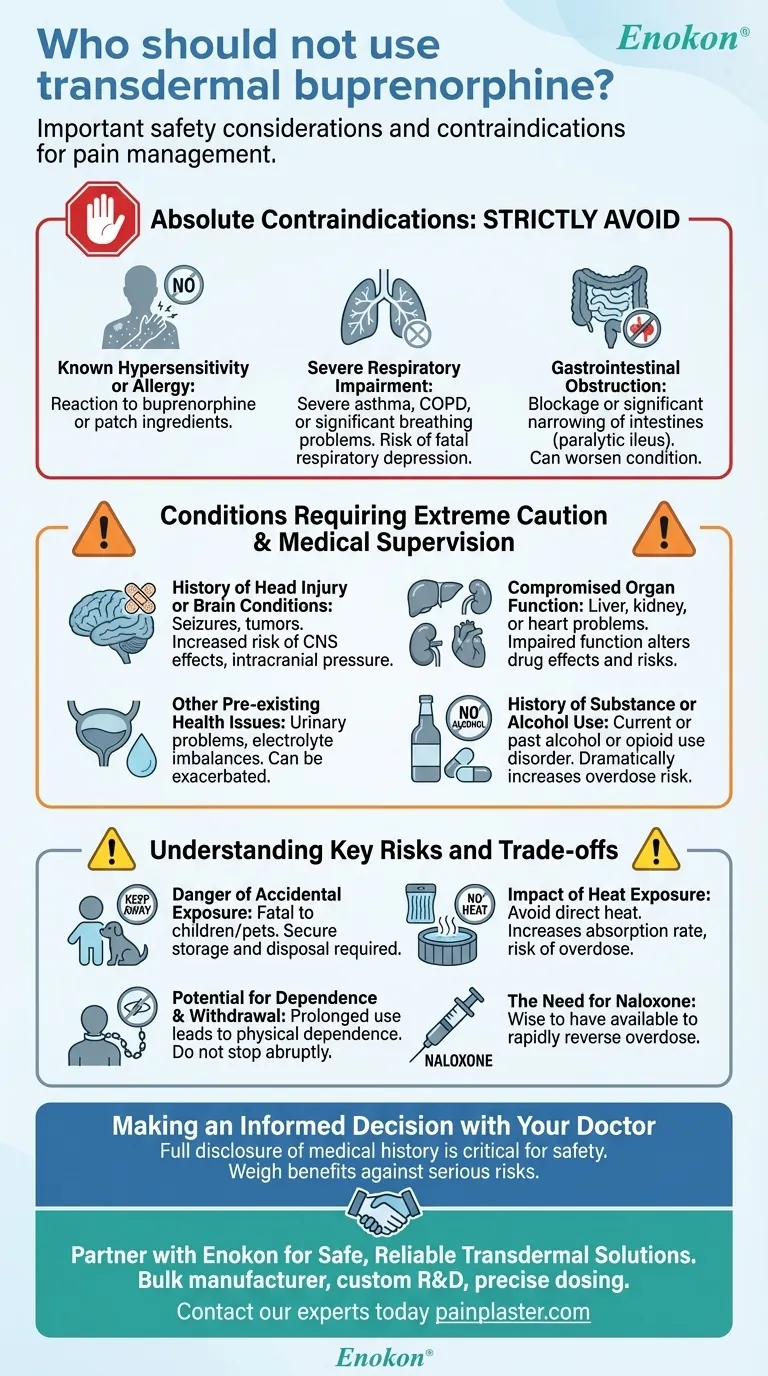
Transdermal buprenorphine is a highly effective medication for managing chronic pain, but it is not a safe option for everyone. Specifically, individuals with a known allergy to its ingredients, those with severe respiratory problems like asthma, or people who have a blockage or significant narrowing of their intestines (paralytic ileus) should not use this medication.
The decision to use transdermal buprenorphine hinges on a careful evaluation of your respiratory health, gastrointestinal function, and overall medical history. Absolute contraindications are clear, but numerous other conditions require a frank discussion with your doctor to weigh the benefits against serious potential risks.

Absolute Contraindications: When Buprenorphine Must Be Avoided
Certain conditions present a risk so significant that the use of transdermal buprenorphine is strictly contraindicated. These are non-negotiable safety boundaries.
Known Hypersensitivity or Allergy
If you have ever had an allergic reaction to buprenorphine or any other ingredient in the transdermal patch, you must not use it. An allergic reaction can be severe and life-threatening.
Severe Respiratory Impairment
The primary risk of all opioid medications, including buprenorphine, is respiratory depression—the slowing of breathing. Individuals with severe asthma or other significant breathing problems are at a much higher risk for this dangerous side effect.
Gastrointestinal Obstruction
Buprenorphine can slow down the movement of the gut. In individuals who already have a blockage or narrowing in their intestines, this effect can worsen the condition, leading to a serious medical emergency.
Conditions Requiring Extreme Caution and Medical Supervision
Beyond absolute contraindications, many pre-existing conditions demand a thorough risk assessment by a qualified physician. Full disclosure of your medical history is critical for your safety.
History of Head Injury or Brain Conditions
Patients with a history of head injuries, seizures, or brain tumors may be more susceptible to the central nervous system effects of buprenorphine. The medication can increase intracranial pressure and obscure the clinical course of a head injury.
Compromised Organ Function
Your body's ability to process and clear the medication is crucial. A history of liver, kidney, or heart problems must be reported, as impaired organ function can alter the drug's effects and increase the risk of adverse events.
Other Pre-existing Health Issues
Conditions like urinary problems or electrolyte imbalances also need to be discussed with your doctor. These can be exacerbated or complicate treatment with buprenorphine.
History of Substance or Alcohol Use
Inform your doctor about any current or past history of alcohol use or opioid use disorder. Combining buprenorphine with other central nervous system depressants like alcohol dramatically increases the risk of overdose and respiratory depression.
Understanding the Key Risks and Trade-offs
Safe use of transdermal buprenorphine requires understanding its inherent risks. These factors are central to the conversation you must have with your healthcare provider.
The Danger of Accidental Exposure
The patch contains a powerful medication that can be fatal to a child or pet who chews on or ingests it. Patches must be stored, applied, and disposed of securely and kept out of reach at all times.
The Impact of Heat Exposure
Do not expose the patch to direct heat sources like heating pads, electric blankets, or hot tubs. Heat can increase the rate at which the medication is absorbed into your body, potentially leading to an overdose.
Potential for Dependence and Withdrawal
Like all opioids, prolonged use of buprenorphine can lead to physical dependence. You should never stop using the medication abruptly without medical guidance, as this can cause withdrawal symptoms.
The Need for Naloxone
Because of the risk of accidental overdose, it is wise to have naloxone available. This is a medication that can rapidly reverse an opioid overdose and should be on hand for you or a household member to administer in an emergency.
Making an Informed Decision with Your Doctor
The safe use of transdermal buprenorphine depends entirely on a transparent partnership between you and your healthcare provider.
- If your primary focus is managing pain with a history of breathing problems: You must prioritize a discussion on the significant risk of respiratory depression and explore all alternatives.
- If your primary focus is managing pain with a complex medical history: Your key takeaway is that full disclosure of every condition—from head injuries to organ function—is non-negotiable for safe treatment.
- If your primary focus is safety for yourself and your family: You must commit to the strict protocols for patch application, heat avoidance, secure storage, and proper disposal to prevent accidental exposure.
Ultimately, an open and honest dialogue with your physician is the only way to ensure this medication helps you without causing harm.
Summary Table:
| Category | Who Should Avoid / Use Extreme Caution |
|---|---|
| Absolute Contraindications | Known allergy to buprenorphine, severe respiratory impairment (e.g., asthma), gastrointestinal obstruction (paralytic ileus). |
| Conditions Requiring Caution | History of head injury/brain conditions, compromised liver/kidney/heart function, urinary problems, electrolyte imbalances, substance/alcohol use disorder. |
| Key Safety Risks | Risk of respiratory depression, accidental exposure (children/pets), heat exposure increasing absorption, physical dependence, need for naloxone availability. |
Partner with Enokon for Safe, Reliable Transdermal Solutions
If you are a healthcare or pharmaceutical distributor or brand developing pain management products, ensuring patient safety is paramount. Enokon is a bulk manufacturer of reliable transdermal patches and pain plasters. Our technical expertise supports custom R&D and development, helping you create formulations that prioritize patient contraindications and safety profiles.
Benefit from our expertise to:
- Develop custom patches with precise dosing and release profiles.
- Leverage our R&D capabilities for formulations that meet specific safety and efficacy needs.
- Access high-quality, bulk-manufactured transdermal delivery systems you can trust.
Contact our experts today to discuss how we can support your transdermal product development with safety and reliability at the core.
Visual Guide
Related Products
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Far Infrared Knee Pain Patch Heat Patches for Pain Relief
- Far Infrared Pain Patch Relief Pain Reliever for Back
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Asthma Cough and Pain Relief Patch for Adults and Kids
People Also Ask
- How do pain relief patches compare to other pain relief methods? Discover Targeted, Long-Lasting Relief
- How should pain relief patches be applied and used? A Guide to Safe & Effective Targeted Relief
- How often should pain relief patches be used? Get the Right Schedule for Targeted Relief
- How quickly does the Deep Heat Pain Relief Back Patch activate and how long does it provide warmth? Get 16-Hour Relief
- How does the Deep Heat Back Patch work? A Drug-Free Solution for Targeted Pain Relief















