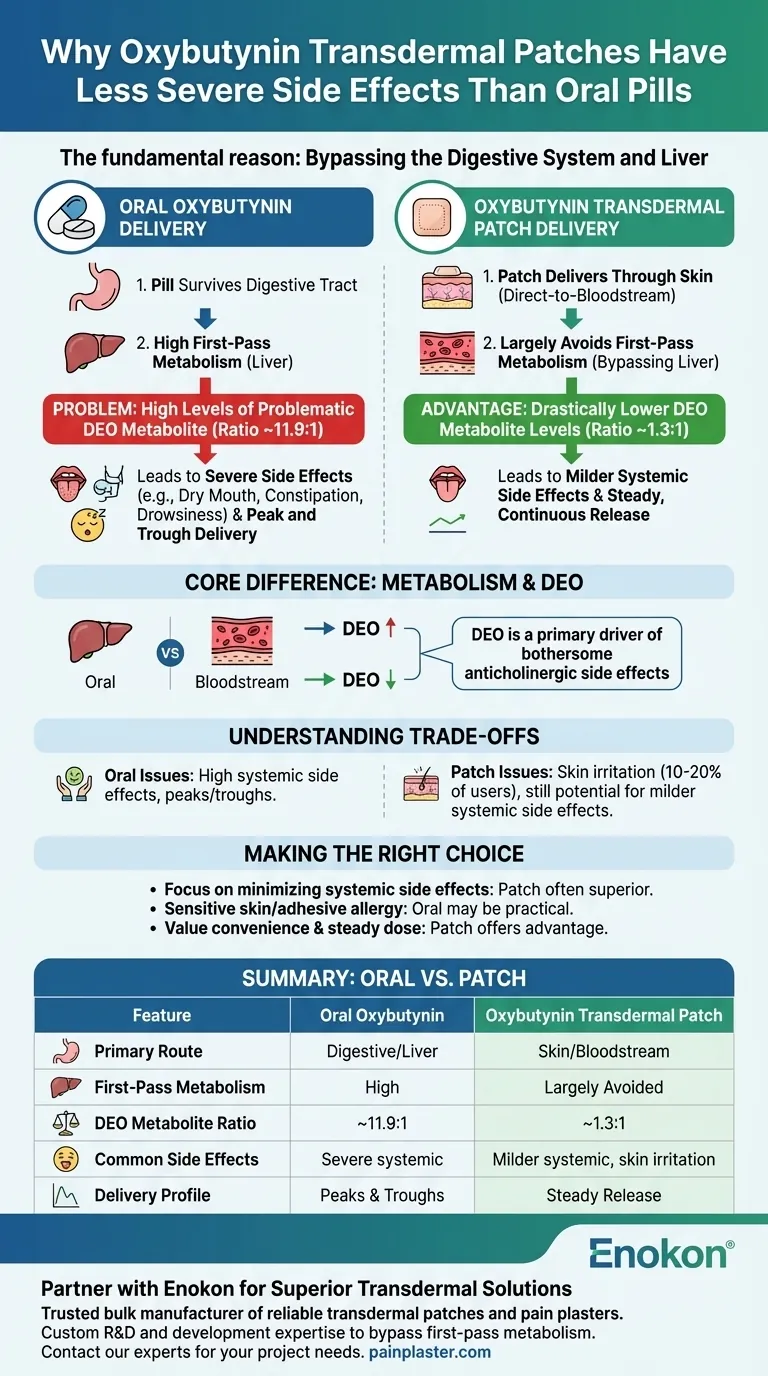
The fundamental reason is that the transdermal patch delivers oxybutynin directly through the skin into your bloodstream, which largely bypasses your digestive system and liver. This direct-to-bloodstream route dramatically changes how the drug is processed, leading to a more favorable side effect profile for many users compared to the oral pill.
The core difference lies in metabolism. Oral oxybutynin is heavily processed by the liver, creating a high concentration of a metabolite that causes many of the drug's most bothersome side effects. The patch avoids this "first-pass" liver metabolism, resulting in lower levels of this metabolite and, consequently, fewer systemic side effects.
How Oral Delivery Creates Side Effects
When you take a pill, it doesn't go directly to where it's needed. It first has to survive a complex journey through your digestive tract and liver, a process that significantly alters the drug.
The "First-Pass" Metabolism Effect
An oral oxybutynin tablet is absorbed in your gut and sent straight to the liver. The liver acts as the body's primary processing plant and metabolizes a large portion of the drug before it ever reaches the rest of your circulation.
This initial, aggressive breakdown is known as first-pass metabolism.
The Problematic DEO Metabolite
During this first-pass metabolism, the liver converts oxybutynin into several other compounds, most notably a metabolite called N-desethyloxybutynin (DEO).
Studies show that after taking an oral dose, the ratio of this DEO metabolite to the parent drug can be as high as 11.9 to 1. This metabolite is believed to be a primary driver of anticholinergic side effects like severe dry mouth, constipation, and drowsiness.
The Advantage of the Transdermal Route
The transdermal patch was specifically designed to circumvent the problems caused by first-pass metabolism and provide a more stable and efficient delivery method.
Bypassing the Liver
By absorbing the medication through the skin, the patch allows oxybutynin to enter the general bloodstream directly. This lets it circulate throughout the body and reach its target before it makes its first pass through the liver.
Drastically Lower Metabolite Levels
Because the patch largely avoids this initial liver breakdown, it produces far lower levels of the problematic DEO metabolite. The ratio of DEO to the parent drug with the patch is only about 1.3 to 1, a stark contrast to the oral formulation.
This dramatic reduction in DEO is the principal reason why systemic side effects are often less frequent and less severe with the patch.
Stable and Continuous Delivery
A pill delivers a spike of medication that peaks and then falls, creating fluctuations in both therapeutic effect and side effects.
The patch, in contrast, provides a slow, continuous, and steady release of oxybutynin. This stability helps to minimize the "peak and trough" effect, which can further contribute to a better-tolerated experience.
Understanding the Trade-offs of the Patch
While the patch often reduces systemic side effects, it is not without its own set of challenges. The primary trade-off is moving the source of irritation from internal to external.
The Issue of Skin Irritation
The most common complaint with the oxybutynin patch is application site reactions. Between 10 to 20 percent of users experience some form of skin irritation, such as redness, itching, or burning.
This issue is significant enough that approximately one in ten patients will discontinue using the patch specifically because of these skin-related symptoms.
Systemic Side Effects Can Still Occur
"Less severe" does not mean "non-existent." Users of the patch can still experience classic anticholinergic side effects, including dry mouth, constipation, dizziness, blurred vision, and drowsiness, though they are often milder.
Serious but Uncommon Risks
Both oral and transdermal forms carry rare but serious risks that require immediate medical attention. These can include allergic reactions like hives or swelling, difficulty breathing, sudden eye pain, or an inability to urinate.
Making the Right Choice for Your Goal
Your choice between the oral and transdermal forms of oxybutynin depends on balancing the potential benefits against the distinct side effect profiles.
- If your primary focus is minimizing systemic side effects like severe dry mouth and drowsiness: The transdermal patch is often the superior choice due to its ability to bypass liver metabolism.
- If you have very sensitive skin or a known allergy to adhesives: The oral formulation may be the more practical option, even with its higher risk of systemic side effects.
- If you value convenience and a steady, continuous dose: The patch offers a clear advantage over the need to remember daily pills and avoids the "peak and trough" cycle of medication levels.
Understanding these delivery mechanisms empowers you to have a more informed discussion with your healthcare provider about the best therapeutic option for you.
Summary Table:
| Feature | Oral Oxybutynin | Oxybutynin Transdermal Patch |
|---|---|---|
| Primary Route | Digestive System & Liver | Directly through Skin (Bloodstream) |
| First-Pass Metabolism | High | Largely Avoided |
| DEO Metabolite Ratio | ~11.9:1 | ~1.3:1 |
| Common Side Effects | Severe dry mouth, constipation, drowsiness | Milder systemic side effects, potential skin irritation |
| Delivery Profile | Peaks and troughs | Steady, continuous release |
Partner with Enokon for Superior Transdermal Solutions
As a trusted bulk manufacturer of reliable transdermal patches and pain plasters, Enokon helps healthcare and pharmaceutical brands deliver better patient outcomes. Our technical expertise in custom R&D and development ensures your products effectively bypass first-pass metabolism, minimizing side effects and maximizing patient comfort.
Benefit from our capabilities:
- Custom Formulation: Tailor patch delivery systems to your specific API.
- Technical Expertise: Leverage our deep knowledge of transdermal technology.
- Reliable Manufacturing: Scale production with consistent, high-quality results.
Ready to develop a more tolerable and effective transdermal product? Contact our experts today to discuss your project needs.
Visual Guide
Related Products
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Heating Pain Relief Patches for Menstrual Cramps
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Menthol Gel Pain Relief Patch
- Mugwort Wormwood Pain Relief Patch for Neck Pain
People Also Ask
- How does the Deep Heat Back Patch work? A Drug-Free Solution for Targeted Pain Relief
- Can heat patches be used for fresh injuries? Avoid This Common Mistake for Faster Recovery
- What types of pain can the Deep Heat Pain Relief Back Patch be used for? Targeted Relief for Muscles & Joints
- How quickly does the Deep Heat Pain Relief Back Patch activate and how long does it provide warmth? Get 16-Hour Relief
- What did the UK Million Women Study find regarding transdermal versus oral hormone therapy? A Safer Choice for Gallbladder Health

















