Informing your healthcare provider about all medications you take is a critical safety measure when using transdermal buprenorphine. This is because many other common drugs, supplements, and even foods can interfere with how your body processes buprenorphine, altering its concentration in your bloodstream. These interactions can either render the pain medication ineffective or, more dangerously, increase its levels to a point that causes serious side effects.
The core issue is drug metabolism. Other substances can change the speed at which your body breaks down buprenorphine, leading to unpredictable and potentially unsafe blood levels. Full disclosure allows your provider to anticipate these interactions and adjust your treatment to ensure it remains both effective and safe.

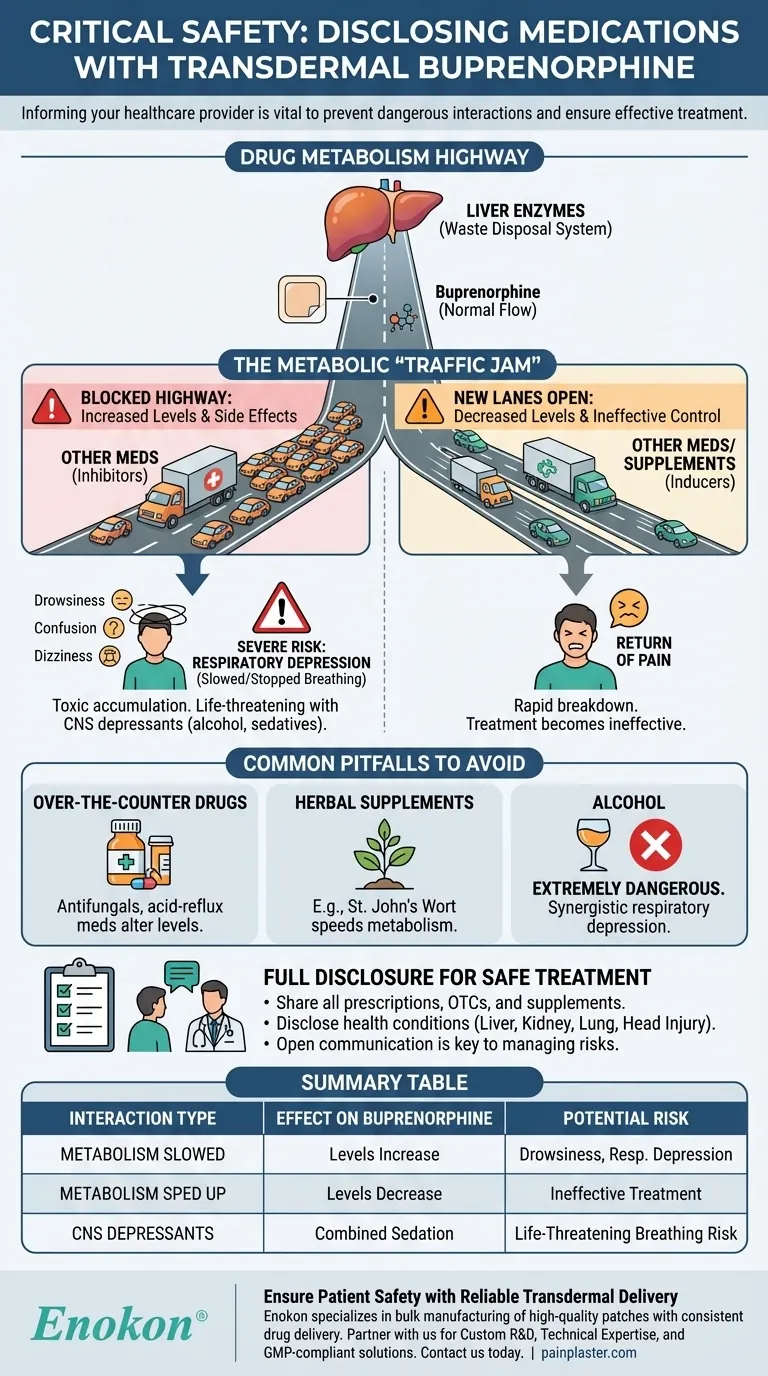
The Central Role of Drug Metabolism
How Your Body Processes Buprenorphine
Your liver contains specific enzymes that act like a waste disposal system, breaking down medications like buprenorphine so they can be removed from your body. This process happens at a predictable rate, which is how your doctor determines the correct patch strength for you.
An Analogy: The Metabolic Highway
Think of these liver enzymes as a single-lane highway. Buprenorphine is one of the cars traveling on it. When you take another medication that uses the same "highway," you can create a traffic jam.
Two Primary Interaction Risks
This metabolic "traffic jam" can go one of two ways. Some drugs block the highway, causing buprenorphine levels to build up. Others can open new lanes, causing buprenorphine to be cleared out too quickly.
The Consequences of Altered Buprenorphine Levels
The Risk of Ineffective Pain Control
If another drug or supplement speeds up buprenorphine metabolism, the level of medication in your blood will drop. This can lead to a return of your pain, defeating the purpose of the treatment.
The Danger of Increased Side Effects
Conversely, if another medication slows down metabolism, buprenorphine can accumulate in your body to toxic levels. This significantly increases the risk of side effects like extreme drowsiness, confusion, and dizziness.
Severe Risk: Respiratory Depression
The most serious danger is respiratory depression—dangerously slow or stopped breathing. As an opioid, high levels of buprenorphine can suppress the body's instinct to breathe, especially when combined with other central nervous system depressants like alcohol, sedatives, or other opioids. This is a life-threatening emergency.
Common Pitfalls to Avoid
Over-the-Counter Medications Matter
Do not assume that non-prescription drugs are harmless. Common antifungals or acid-reflux medications can significantly alter buprenorphine levels.
The Impact of Herbal Supplements
Herbal products are not benign. For example, St. John's Wort is a well-known supplement that can speed up drug metabolism, potentially making your buprenorphine patch ineffective.
Alcohol is a Major Risk Factor
Combining buprenorphine with alcohol is extremely dangerous. Both substances suppress the central nervous system, and their combined effect on slowing your breathing is much greater than either one alone.
Disclosing Your Full Health History
Your provider also needs to know about underlying health conditions. Problems with your liver, kidneys, or lungs, or a history of head injury or seizures, can change how your body handles the medication, making full disclosure of your health history as vital as your medication list.
Making the Right Choice for Your Goal
To ensure your treatment is managed correctly, open communication is key.
- If your primary focus is effective pain management: Ensure your provider knows about any substance that might speed up buprenorphine's breakdown, making your patch less effective.
- If your primary focus is avoiding dangerous side effects: You must disclose all other medications, supplements, and alcohol use that could slow metabolism and lead to dangerously high drug levels.
- If your primary focus is overall well-being: Maintain a complete, updated list of all prescriptions, over-the-counter drugs, supplements, and health conditions to share at every medical appointment.
Transparent communication with your healthcare team is the single most important factor in using transdermal buprenorphine safely and successfully.
Summary Table:
| Interaction Type | Effect on Buprenorphine | Potential Risk |
|---|---|---|
| Metabolism Slowed | Levels increase in bloodstream | Drowsiness, dizziness, respiratory depression |
| Metabolism Sped Up | Levels decrease in bloodstream | Return of pain, ineffective treatment |
| CNS Depressants (e.g., alcohol) | Combined sedative effect | Severely slowed breathing, life-threatening risk |
Ensure Patient Safety with Reliable Transdermal Delivery
As a healthcare distributor or brand, managing medication safety is paramount. Enokon specializes in the bulk manufacturing of high-quality, reliable transdermal patches, including pain plasters. Our technical expertise ensures consistent drug delivery, a critical factor in mitigating interaction risks.
Partner with us for:
- Custom R&D: Develop patches tailored to specific drug profiles and patient needs.
- Technical Expertise: Leverage our knowledge to optimize delivery systems for safety and efficacy.
- Bulk Manufacturing: Access a trusted supply of GMP-compliant transdermal solutions.
Let's collaborate to create safer pain management solutions. Contact our team today to discuss your project requirements.
Visual Guide
Related Products
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Far Infrared Pain Patch Relief Pain Reliever for Back
- Far Infrared Knee Pain Patch Heat Patches for Pain Relief
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Asthma Cough and Pain Relief Patch for Adults and Kids
People Also Ask
- How effective are pain relief patches for muscle pain? Target Localized Pain with Transdermal Delivery
- How does the Deep Heat Back Patch work? A Drug-Free Solution for Targeted Pain Relief
- How do pain relief patches provide targeted relief? Discover the Science Behind Effective Pain Management
- How quickly does the Deep Heat Pain Relief Back Patch activate and how long does it provide warmth? Get 16-Hour Relief
- How do Deep Heat Pain Relief Patches provide pain relief? Discover the Drug-Free Mechanism














