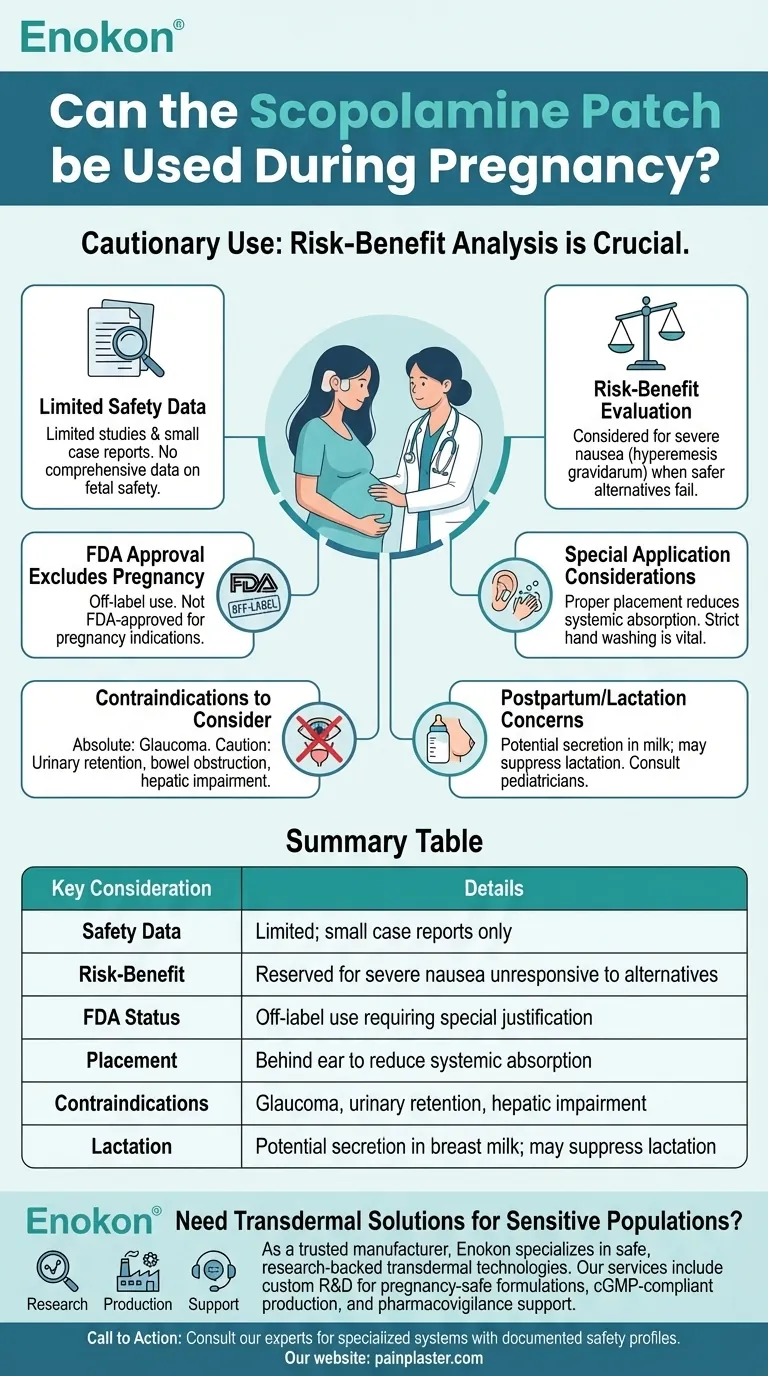
The scopolamine patch should be used with caution during pregnancy due to limited safety data. While it's FDA-approved for motion sickness and post-surgical nausea in non-pregnant populations, its use in pregnancy is considered only when benefits outweigh potential risks—typically reserved for severe nausea/vomiting cases unresponsive to safer alternatives. Healthcare providers must evaluate individual circumstances, as systemic absorption could theoretically affect fetal development. Pregnant women should never self-prescribe this medication and must consult their OB/GYN or maternal-fetal medicine specialist before use.

Key Points Explained:
-
Limited Safety Data in Pregnancy
- No comprehensive studies establish absolute safety for fetal development
- Existing data comes from small case reports rather than controlled trials
- Unknown risks regarding potential teratogenic effects or pregnancy complications
-
Risk-Benefit Evaluation Required
- May be considered for hyperemesis gravidarum when standard treatments fail
- Requires careful assessment of maternal health vs. theoretical fetal risks
- Alternative therapies like vitamin B6/doxylamine combinations should be tried first
-
FDA Approval Excludes Pregnancy
- Current indications: motion sickness (adults/children >12), post-anesthesia nausea, vestibular disorders
- Pregnancy constitutes off-label use requiring special justification
- Different safety profile than anti inflammatory patch medications
-
Special Application Considerations
- Proper placement behind ear reduces systemic absorption
- Hand washing after application prevents accidental ocular exposure
- Monitoring for anticholinergic side effects (dry mouth, blurred vision) is crucial
-
Contraindications to Consider
- Absolute contraindication in glaucoma patients
- Caution with urinary retention or bowel obstruction histories
- Requires dose adjustment in hepatic impairment
-
Postpartum/Lactation Concerns
- Potential secretion in breast milk (anticholinergic effects on infant)
- May suppress lactation due to antimuscarinic properties
- Requires pediatric consultation if breastfeeding while using
Healthcare providers must document informed consent discussions regarding unknown long-term neurodevelopmental outcomes when using scopolamine patches during pregnancy. The decision should involve multidisciplinary teams including pharmacists and neonatologists for high-risk cases.
Summary Table:
| Key Consideration | Details |
|---|---|
| Safety Data | Limited studies; small case reports only |
| Risk-Benefit | Reserved for severe nausea unresponsive to alternatives |
| FDA Status | Off-label use requiring special justification |
| Placement | Behind ear to reduce systemic absorption |
| Contraindications | Glaucoma, urinary retention, hepatic impairment |
| Lactation | Potential secretion in breast milk; may suppress lactation |
Need transdermal solutions tailored for sensitive populations?
As a trusted manufacturer of medical patches, Enokon specializes in safe, research-backed transdermal technologies for healthcare brands and distributors. Our team offers:
- Custom R&D for pregnancy-safe formulations
- cGMP-compliant production of therapeutic patches
- Pharmacovigilance support for special populations
Consult our experts about developing or sourcing specialized transdermal systems with documented safety profiles.
Visual Guide
Related Products
- Prostate Pain Kidney Health Care Patch for Men
- Asthma Cough and Pain Relief Patch for Adults and Kids
- Capsaicin Chili Medicated Pain Relief Patches
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Herbal Eye Protection Patch Eye Patch
People Also Ask
- What should be done if a testosterone patch is missed or falls off? Follow these simple timing rules for safety and consistency.
- What is the purpose of testosterone patches? A Steady Solution for Low Testosterone
- What should be done before undergoing an MRI while using testosterone patches? Remove it to prevent serious burns.
- What should be done if a dose of testosterone patches is missed? Regain Stability and Safety
- How often should testosterone patches be applied? Daily Dosage & Best Practices














