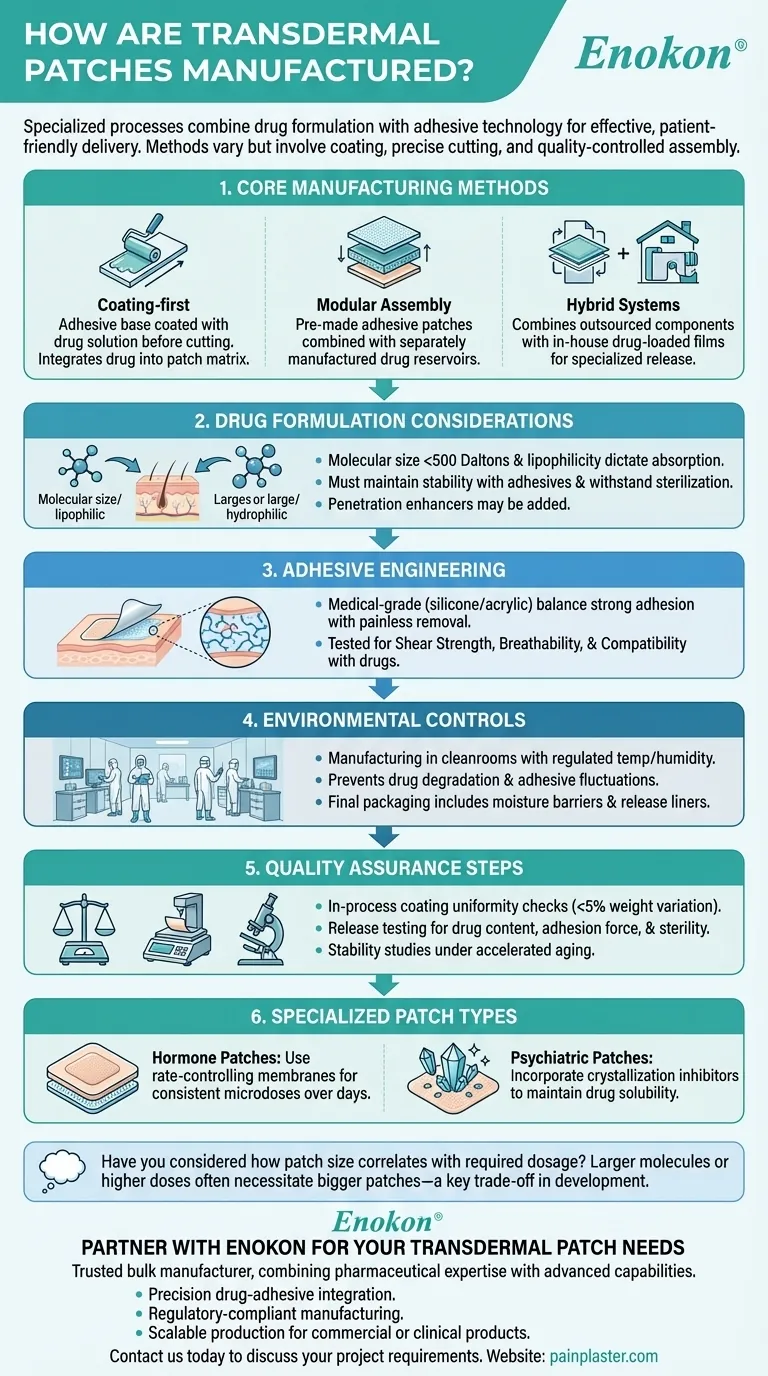
Transdermal patches are manufactured through specialized processes that combine drug formulation with adhesive technology to create effective, patient-friendly delivery systems. The manufacturing methods vary based on patch design and drug properties, but generally involve coating drug solutions onto adhesive materials, precise cutting, and quality-controlled assembly. These patches offer advantages like bypassing digestive systems, providing steady dosing, and improving compliance, but require careful consideration of molecular properties and skin compatibility during production.

Key Points Explained:
-
Core Manufacturing Methods
- Coating-first approach: The adhesive base material is coated with the drug solution before being cut into individual patches. This integrates the drug directly into the patch matrix.
- Modular assembly: Pre-made adhesive patches (outsourced or in-house) are combined with separately manufactured drug reservoirs or layers. This allows customization of drug-loading capacity.
- Hybrid systems: Combine outsourced adhesive components with in-house production of drug-loaded films or membranes, enabling specialized release mechanisms like transdermal patch rate control membranes.
-
Drug Formulation Considerations
- Molecular size (<500 Daltons penetrates best) and lipophilicity (fat-soluble drugs absorb better) dictate absorption rates.
- Solutions must maintain stability when combined with adhesives and withstand sterilization processes.
- Additives like penetration enhancers (e.g., alcohols) may be incorporated to improve skin permeability.
-
Adhesive Engineering
- Medical-grade adhesives must balance strong skin adhesion with painless removal, often using silicone or acrylic polymers.
- Adhesives are tested for:
- Shear strength (resistance to peeling from movement/sweat)
- Breathability to prevent skin maceration
- Compatibility with drug molecules to prevent inactivation
-
Environmental Controls
- Manufacturing occurs in cleanrooms with regulated temperature/humidity to prevent:
- Drug degradation (heat/moisture sensitivity)
- Adhesive performance fluctuations
- Final packaging often includes moisture barriers and protective release liners.
- Manufacturing occurs in cleanrooms with regulated temperature/humidity to prevent:
-
Quality Assurance Steps
- In-process checks of coating uniformity (e.g., weight variation <5%)
- Release testing for drug content, adhesion force, and sterility
- Stability studies under accelerated aging conditions
-
Specialized Patch Types
- Hormone patches: Use rate-controlling membranes to deliver consistent microdoses over days.
- Psychiatric patches (e.g., asenapine): Incorporate crystallization inhibitors to maintain drug solubility in the adhesive matrix.
Have you considered how patch size correlates with the drug's required daily dosage? Larger molecules or higher doses often necessitate bigger patches, impacting wearability and patient acceptance—a key trade-off manufacturers optimize during development. These discreet systems exemplify how material science and pharmacology converge to create technologies that simplify complex treatment regimens.
Summary Table:
| Key Aspect | Details |
|---|---|
| Core Methods | Coating-first, modular assembly, hybrid systems |
| Drug Formulation | Molecular size <500 Da, lipophilicity, stability with adhesives |
| Adhesive Engineering | Silicone/acrylic polymers, shear strength, breathability |
| Environmental Controls | Cleanroom production, moisture/packaging barriers |
| Quality Assurance | Coating uniformity, adhesion force, sterility, stability testing |
| Specialized Patch Types | Hormone (rate-controlled), psychiatric (crystallization inhibitors) |
Partner with Enokon for Your Transdermal Patch Needs
As a trusted bulk manufacturer of transdermal patches and pain plasters, we combine pharmaceutical expertise with advanced production capabilities to deliver reliable, customizable solutions. Whether you're a healthcare distributor or a brand seeking tailored R&D support, our team ensures:
- Precision drug-adhesive integration for optimal delivery
- Regulatory-compliant manufacturing in controlled environments
- Scalable production for commercial or clinical-stage products
Contact us today to discuss your project requirements and leverage our technical mastery in transdermal systems.
Visual Guide
Related Products
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Herbal Eye Protection Patch Eye Patch
- Heating Pain Relief Patches for Menstrual Cramps
- Menthol Gel Pain Relief Patch
- Icy Hot Menthol Medicine Pain Relief Patch
People Also Ask
- Can heat patches be used for fresh injuries? Avoid This Common Mistake for Faster Recovery
- Are heat patches safe for all body parts? Key Safety Zones and No-Go Areas Explained
- What are the key features of the Deep Heat Pain Relief Back Patch? Get Up to 16 Hours of Drug-Free Relief
- What did the UK Million Women Study find regarding transdermal versus oral hormone therapy? A Safer Choice for Gallbladder Health
- How do Deep Heat Pain Relief Patches provide pain relief? Discover the Drug-Free Mechanism














