At its core, the oxybutynin transdermal matrix system is a sophisticated skin patch that continuously delivers medication through the skin to relax the bladder muscle. It consists of a multi-layer patch containing the drug oxybutynin, which slowly diffuses from an adhesive matrix, through the skin's outer layers, and into the bloodstream at a controlled rate over several days.
The true innovation of the oxybutynin patch isn't just the drug itself, but its delivery method. The system is engineered to provide a constant, steady dose directly to the body, bypassing the digestive system to effectively treat overactive bladder while minimizing the peaks and troughs common with oral medication.

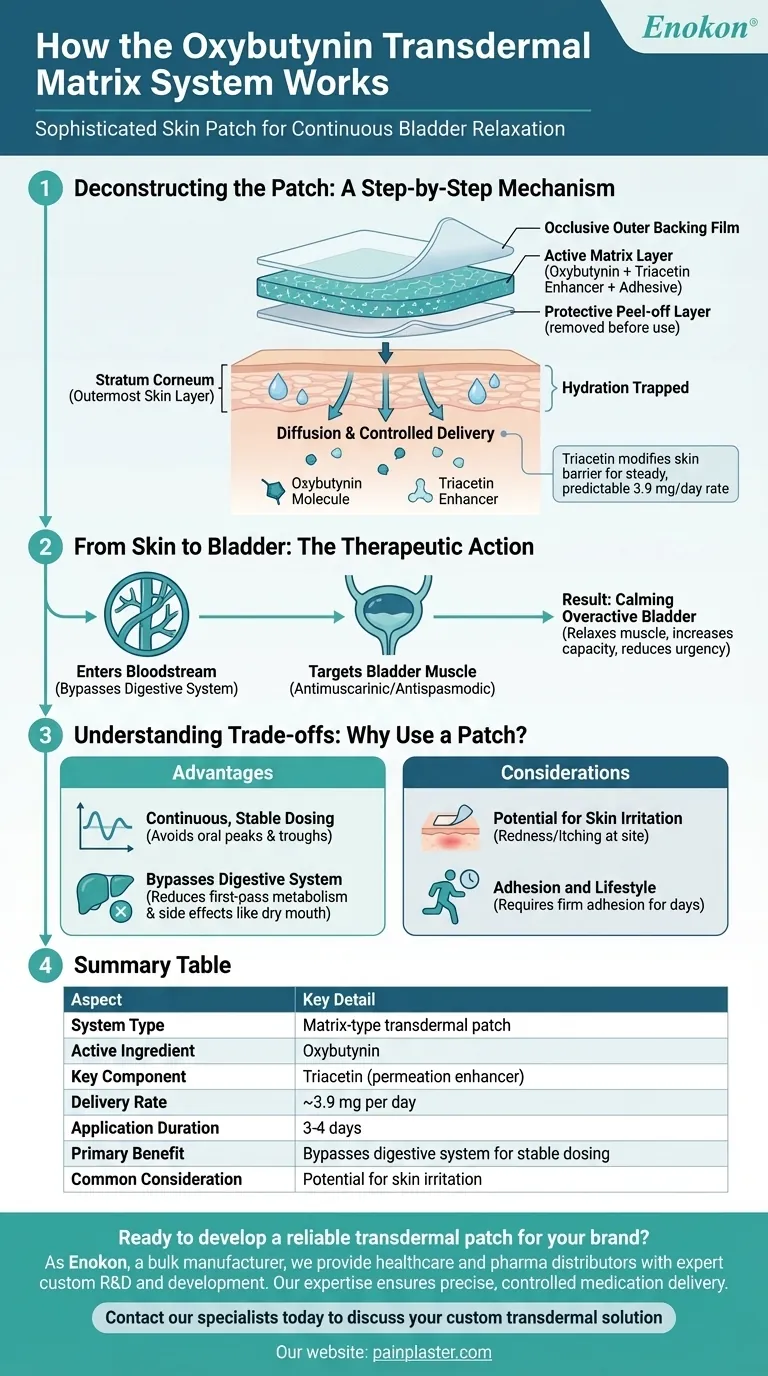
Deconstructing the Patch: A Step-by-Step Mechanism
The effectiveness of the oxybutynin transdermal system comes from its precise physical construction and its interaction with your skin.
The Physical Layers
The patch is a matrix-type system, meaning the drug is evenly mixed into the adhesive layer. It has three parts: a protective peel-off layer you remove, an occlusive outer backing film that prevents the drug from evaporating, and the active matrix layer containing oxybutynin, an acrylic adhesive, and a permeation enhancer called triacetin.
The Initial Application
When you apply the patch to the skin (typically the abdomen, hip, or buttock), the occlusive backing film traps moisture. This hydrates the outermost layer of your skin, the stratum corneum, making it more permeable to the medication.
Diffusion into the Skin
With the skin hydrated, the drug begins to move. Following the principle of diffusion, oxybutynin and the triacetin enhancer travel from the high-concentration patch matrix into the lower-concentration environment of your skin.
Controlling the Delivery Rate
The permeation enhancer (triacetin) is critical for controlling the dose. It interacts with the natural lipids in your skin, modifying the skin barrier just enough to allow oxybutynin to pass through at a steady, predictable rate of about 3.9 mg per day.
From Skin to Bladder: The Therapeutic Action
Once the drug clears the skin barrier, it begins its therapeutic work.
Entering the Bloodstream
After passing through the skin, oxybutynin is absorbed by the small blood vessels underneath and enters the systemic circulation. This allows the drug to travel throughout your body to its target.
Targeting the Bladder Muscle
Oxybutynin is an antimuscarinic and antispasmodic agent. It works by blocking certain nerve impulses to the smooth muscle of the bladder, causing it to relax.
The Result: Calming an Overactive Bladder
By relaxing these muscles, the patch decreases uninhibited bladder contractions. This action increases the bladder's capacity to hold urine, delays the initial urge to void, and reduces the frequency and urgency associated with an overactive bladder.
Understanding the Trade-offs: Why Use a Patch?
Choosing a transdermal system over an oral pill involves a distinct set of advantages and considerations. This delivery method is specifically designed to solve problems inherent in oral administration.
Advantage: Continuous, Stable Dosing
Unlike pills, which can create peaks and valleys in medication levels, the patch delivers a continuous and stable dose over its 3-4 day application period. This consistency can lead to more reliable symptom control.
Advantage: Bypassing the Digestive System
When oxybutynin is taken orally, it undergoes extensive "first-pass metabolism" in the liver, which can break down the drug and create byproducts linked to side effects. The patch delivers the drug directly into the bloodstream, bypassing this process and potentially reducing side effects like dry mouth.
Consideration: Potential for Skin Irritation
The most common trade-off is the potential for local skin reactions. The adhesive, the drug itself, or the occlusive environment under the patch can cause redness or itching at the application site.
Consideration: Adhesion and Lifestyle
For the patch to work correctly, it must remain firmly adhered to the skin for several days. This requires careful application and may be a consideration for individuals with very active lifestyles or those with sensitivities to adhesives.
Making the Right Choice for Your Goal
The oxybutynin transdermal system offers a unique approach to managing overactive bladder. Understanding its specific benefits helps you and your doctor decide if it aligns with your treatment goals.
- If your primary focus is consistent symptom control: The patch's steady, continuous drug delivery is designed to avoid the fluctuations that can occur between oral doses.
- If your primary focus is minimizing side effects like dry mouth: The transdermal route bypasses the liver's initial metabolism of the drug, which is a key reason it may be better tolerated than oral forms.
- If your primary focus is convenience: A patch that only needs to be changed twice a week can be much simpler to manage than a daily pill regimen.
Ultimately, this system leverages advanced drug delivery science to provide a targeted and stable solution for managing an overactive bladder.
Summary Table:
| Aspect | Key Detail |
|---|---|
| System Type | Matrix-type transdermal patch |
| Active Ingredient | Oxybutynin |
| Key Component | Triacetin (permeation enhancer) |
| Delivery Rate | ~3.9 mg per day |
| Application Duration | 3-4 days |
| Primary Benefit | Bypasses digestive system for stable dosing |
| Common Consideration | Potential for skin irritation at application site |
Ready to develop a reliable transdermal patch for your brand?
As Enokon, a bulk manufacturer of reliable transdermal patches and pain plasters, we provide healthcare and pharma distributors and brands with expert custom R&D and development services. Our technical expertise ensures your product delivers precise, controlled medication delivery.
Contact our specialists today to discuss your custom transdermal solution.
Visual Guide
Related Products
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Asthma Cough and Pain Relief Patch for Adults and Kids
- Heating Pain Relief Patches for Menstrual Cramps
- Capsaicin Chili Medicated Pain Relief Patches
People Also Ask
- How should the estrogen and progestin contraceptive patch be used? A Guide to Correct Application & Timing
- How does a transdermal estrogen patch differ from oral preparations? A Safer, Steadier Delivery Method
- What are the advantages of using the birth control patch? Enjoy Weekly Convenience & Health Benefits
- What are the potential side effects of testosterone patches? Risks & Safety Guide
- What are the main factors to consider when choosing between the contraceptive patch and the birth control pill? Weighing Convenience vs. Hormone Exposure
- What should someone do if they experience uncomfortable side effects from the birth control patch? A Guide to Navigating Your Health
- What is transdermal estradiol used for? A Guide to Managing Menopause & Bone Health
- What medical conditions can patches treat? Discover the Versatility of Transdermal Drug Delivery
















