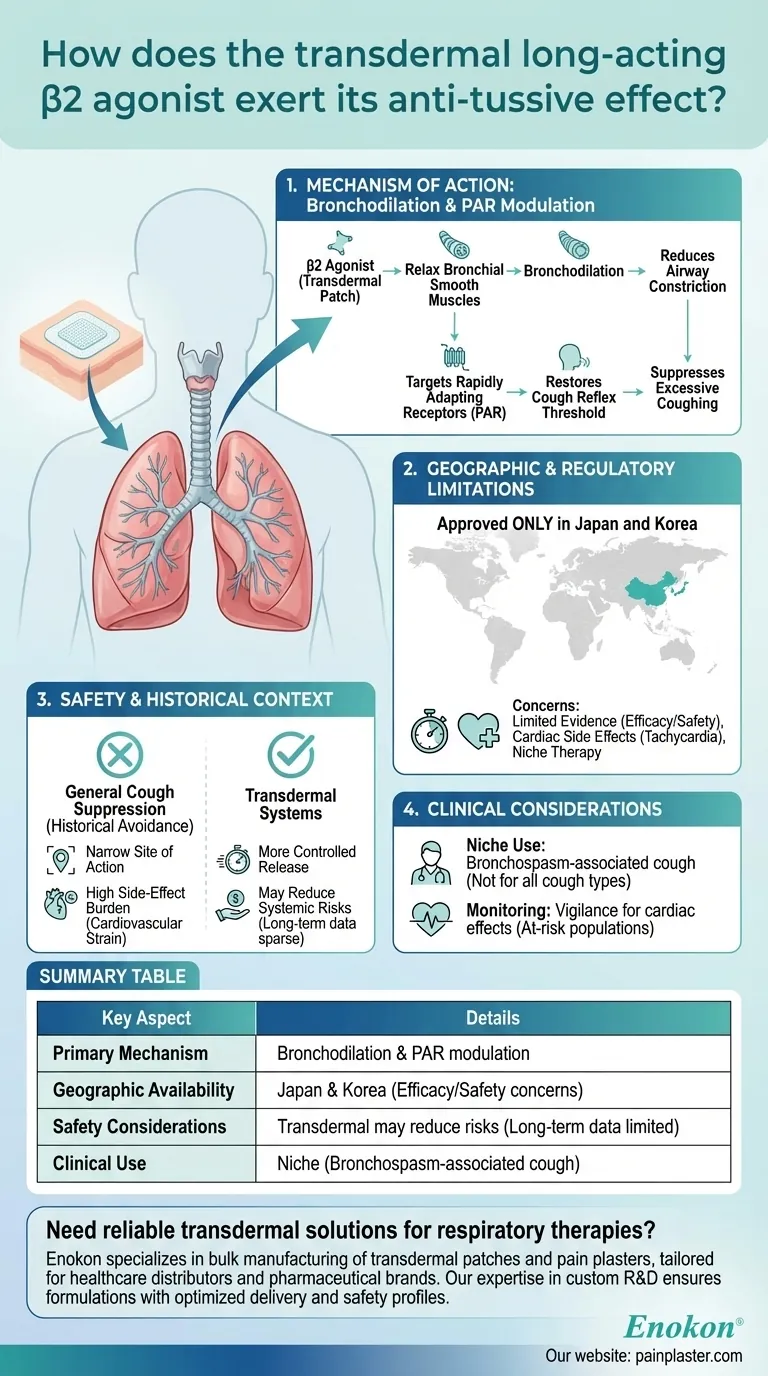
The transdermal long-acting β2 agonist exerts its anti-tussive effect primarily through bronchodilation, acting on rapidly adapting receptors (PAR) to restore the cough reflex threshold. These mechanoreceptors are activated by irritants and bronchospasm, making β2 agonists effective in suppressing cough. However, their use is limited to Japan and Korea due to concerns about efficacy, safety, and cardiac side effects. The transdermal formulation mitigates some systemic risks but remains a niche therapy.
Key Points Explained:
-
Mechanism of Action: Bronchodilation and PAR Modulation
- The drug's primary anti-tussive effect stems from its ability to relax bronchial smooth muscles, reducing airway constriction.
- It specifically targets rapidly adapting receptors (PAR), mechanoreceptors sensitive to irritants and bronchospasm. By restoring the cough reflex threshold of PAR (but not C-fibers), it suppresses excessive coughing.
- This selective action distinguishes it from broader anti-tussives, which may affect multiple pathways.
-
Geographic and Regulatory Limitations
- Approved only in Japan and Korea, the transdermal formulation is a regional therapy. This reflects cautious adoption due to:
- Limited evidence for efficacy and safety as a non-specific anti-tussive.
- Cardiac side effects (e.g., tachycardia) from systemic β2-adrenergic stimulation, though transdermal delivery may reduce such risks.
- Approved only in Japan and Korea, the transdermal formulation is a regional therapy. This reflects cautious adoption due to:
-
Safety and Historical Context
- Before transdermal formulations, β2 agonists were avoided for general cough suppression due to:
- Narrow site of action (primarily airways).
- High side-effect burden (e.g., cardiovascular strain).
- Transdermal systems may offer more controlled release, but long-term safety data remain sparse.
- Before transdermal formulations, β2 agonists were avoided for general cough suppression due to:
-
Clinical Considerations
- Niche use: Suitable for patients with bronchospasm-associated cough but not for all cough types.
- Monitoring: Requires vigilance for cardiac effects, especially in at-risk populations.
This targeted approach highlights the balance between therapeutic innovation and pharmacologic constraints in cough management.
Summary Table:
| Key Aspect | Details |
|---|---|
| Primary Mechanism | Bronchodilation & modulation of rapidly adapting receptors (PAR). |
| Geographic Availability | Approved only in Japan and Korea due to efficacy/safety concerns. |
| Safety Considerations | Transdermal delivery may reduce cardiac risks; long-term data limited. |
| Clinical Use | Niche application for bronchospasm-associated cough, not general cough. |
Need reliable transdermal solutions for respiratory therapies?
Enokon specializes in bulk manufacturing of transdermal patches and pain plasters, tailored for healthcare distributors and pharmaceutical brands. Our expertise in custom R&D ensures formulations with optimized delivery and safety profiles. Contact us today to discuss scalable, compliant solutions for your therapeutic needs.
Visual Guide
Related Products
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Herbal Eye Protection Patch Eye Patch
- Heating Pain Relief Patches for Menstrual Cramps
- Icy Hot Menthol Medicine Pain Relief Patch
- Menthol Gel Pain Relief Patch
People Also Ask
- How do Deep Heat Pain Relief Patches provide pain relief? Discover the Drug-Free Mechanism
- How does the Deep Heat Back Patch work? A Drug-Free Solution for Targeted Pain Relief
- Can heat patches be used for fresh injuries? Avoid This Common Mistake for Faster Recovery
- What are the key features of the Deep Heat Pain Relief Back Patch? Get Up to 16 Hours of Drug-Free Relief
- How quickly does the Deep Heat Pain Relief Back Patch activate and how long does it provide warmth? Get 16-Hour Relief














