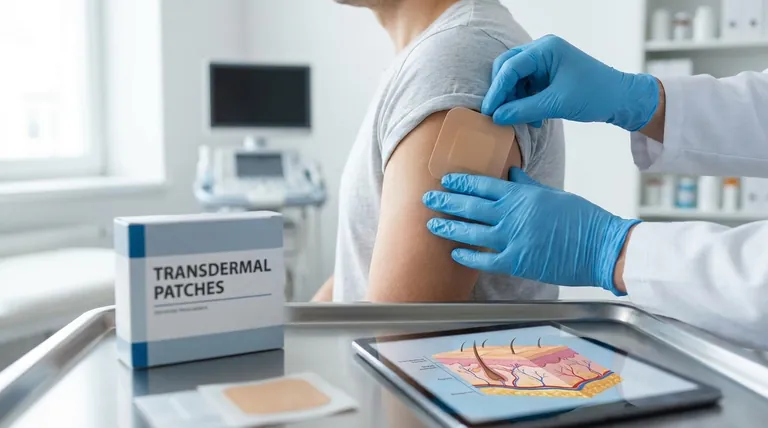
To be direct, the transdermal route dramatically increases fentanyl's bioavailability to approximately 90%. This happens because the skin patch allows the medication to bypass the digestive system and the liver, a process known as first-pass metabolism, which would otherwise break down a significant portion of the drug before it could take effect.
By delivering fentanyl directly into the bloodstream through the skin, the transdermal route ensures a much higher percentage of the drug is active in the body. This allows for lower, more consistent dosing for pain management but also introduces significant risks if not used exactly as prescribed.
The Mechanism: How Transdermal Delivery Works
To understand the impact on bioavailability, it's essential to see how the transdermal patch fundamentally changes the drug's journey into your system compared to an oral pill.
Bypassing the Digestive System
When a drug is taken orally, it must first survive the harsh, acidic environment of the stomach and enzymatic reactions in the intestines. This process can degrade a portion of the medication before it is ever absorbed. The transdermal patch completely avoids this, delivering the drug through the skin.
Avoiding First-Pass Metabolism
The most critical factor is the avoidance of first-pass metabolism. After a drug is absorbed from the gut, it travels directly to the liver, which acts as a filter and metabolizes—or breaks down—a large fraction of it. The transdermal route circumvents the liver, ensuring the drug enters the general bloodstream in a much more complete form.
Direct Entry into Systemic Circulation
With a patch, fentanyl diffuses slowly and continuously through the layers of the skin and into the capillaries. From there, it enters the systemic circulation, the network of blood vessels that carries it throughout the body to the central nervous system, where it binds to mu-receptors to relieve pain.
Understanding the Trade-offs and Risks
The high bioavailability of transdermal fentanyl is a double-edged sword, offering significant therapeutic benefits alongside serious dangers.
Increased Potency and Lower Doses
Because nearly all of the drug in the patch becomes active in the body, clinicians can prescribe much lower doses than would be required orally. This efficiency can help reduce the overall drug load on the body.
The Danger of Overdose
This high efficiency also means there is very little room for error. Changing a patch more frequently than the prescribed 72-hour interval can rapidly introduce a toxic amount of fentanyl into the bloodstream, potentially leading to a fatal overdose.
Loss of Immediate Control
Once applied, the process of absorption is slow and steady. This makes it difficult to quickly adjust the dose up or down, much like trying to speed up or slow down a massive truck. This lack of immediate control is why strict adherence to medical instructions is critical.
Systemic Side Effects Remain
While the patch bypasses the liver on the first pass, the drug still circulates systemically, meaning side effects are still a major concern. Common effects include nausea, constipation, dizziness, and slowed breathing. Severe effects like serotonin syndrome or adrenal insufficiency require immediate medical attention.
Making the Right Choice for Your Goal
Understanding the bioavailability of transdermal fentanyl is key to using it effectively and safely.
- If your primary focus is stable, long-term pain management: The transdermal route's high and consistent bioavailability is a major advantage, providing steady relief without the peaks and troughs of oral dosing.
- If your primary focus is safety: The high bioavailability demands absolute adherence to prescribed schedules, as even small deviations can lead to a dangerous overdose due to the drug's direct and potent entry into the bloodstream.
Ultimately, the transdermal route makes fentanyl a highly effective but unforgiving tool where bioavailability is both its greatest strength and its most significant risk.
Summary Table:
| Factor | Oral Route | Transdermal Route |
|---|---|---|
| Bioavailability | Low (due to first-pass metabolism) | High (~90%) |
| Primary Advantage | - | Stable, continuous pain relief |
| Primary Risk | - | High risk of overdose with misuse |
| Dosing Control | Easier to adjust | Difficult to adjust once applied |
Need a reliable, high-quality transdermal patch for your pain management product line?
As Enokon, a bulk manufacturer of reliable transdermal patches and pain plasters for healthcare and pharmaceutical distributors and brands, we understand the critical importance of precise drug delivery and safety. Our technical expertise ensures custom R&D and development to meet your specific formulation and bioavailability goals.
Let us help you develop a safe and effective transdermal solution. Contact our experts today to discuss your project.
Visual Guide
Related Products
- Capsaicin Chili Medicated Pain Relief Patches
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Icy Hot Menthol Medicine Pain Relief Patch
- Menthol Gel Pain Relief Patch
- Asthma Cough and Pain Relief Patch for Adults and Kids
People Also Ask
- Can pregnant women use pain relief patches? Your Essential Guide to Safe Pain Management
- Are pain relief patches safe for sensitive skin? Your Guide to Safe Use & Skin Testing
- Can the pain relief patch be used with other external analgesic products? A Critical Safety Guide
- How do you apply the Signal Relief patch to find the proper placement? A Step-by-Step Guide to Maximum Relief
- What precautions should be taken with buprenorphine patches? Ensure Safe Use and Avoid Overdose Risks













