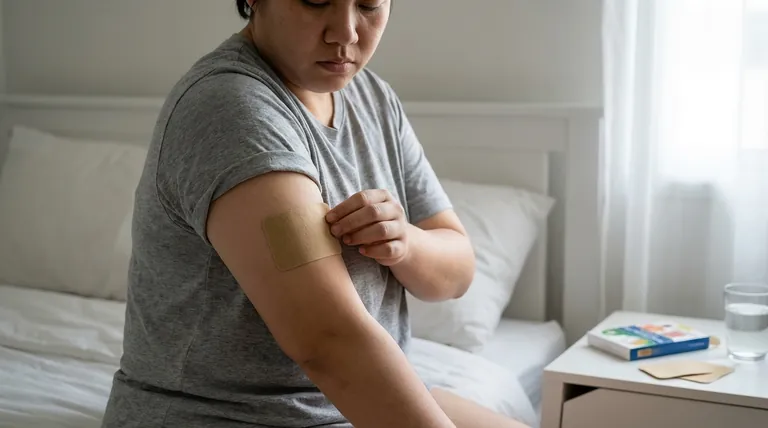
When considering transdermal selegiline for major depression, its effectiveness is modest but statistically significant compared to a placebo. In clinical trials, 33-40% of patients responded to the treatment, whereas response rates for the placebo group were between 22-30%. It is important to note, however, that real-world effectiveness is likely lower than what is observed in these controlled studies.
Transdermal selegiline is a viable treatment option that works by increasing key neurotransmitters in the brain, but its primary limitation is the lack of direct comparison data against more common first-line antidepressants like SSRIs.
How Transdermal Selegiline Works
The Role of MAO Inhibition
Transdermal selegiline belongs to a class of medications known as monoamine oxidase (MAO) inhibitors.
It functions by increasing the levels of certain natural substances in the brain that are essential for maintaining mental and emotional balance. This mechanism is distinct from many other classes of modern antidepressants.
Evaluating Its Clinical Effectiveness
Response Rates in Clinical Trials
The primary evidence for selegiline's effectiveness comes from placebo-controlled trials. These studies show a clear, though not dramatic, advantage for the medication.
The response rate of 33-40% is a key benchmark, but it also means that a majority of patients may not experience a significant clinical response.
Preventing Relapse
Beyond initial treatment, transdermal selegiline is more effective than a placebo at preventing a relapse of depressive symptoms over a one-year period.
However, a notable portion of patients still experience a return of symptoms, with data showing that approximately one in six patients on the medication will relapse within that year.
Understanding the Trade-offs and Limitations
Lack of Comparative Data
A significant gap in the clinical data is the absence of head-to-head trials comparing transdermal selegiline to other standard antidepressants.
This makes it difficult for clinicians and patients to determine its place relative to more widely used treatments, such as SSRIs or SNRIs.
Who Should Not Use It
Transdermal selegiline is not recommended for children or adolescents with depression.
This is due to a known risk of increased suicidal thoughts or attempts in children, teenagers, and young adults taking antidepressant medications.
Practical Application and Dosing
How to Apply the Patch
The patch is designed for once-daily application to clean, dry, and hairless skin.
Approved application sites include the upper torso (chest or back), upper thigh, or the outer surface of the upper arm. It is crucial to rotate the application site each day to avoid skin irritation.
Standard Dosing Protocol
Treatment typically begins at a starting dose of 6 mg per 24 hours.
If a greater clinical response is needed, the dose can be increased in 3 mg increments every two weeks, up to a maximum recommended dose of 12 mg per 24 hours.
Critical Handling Instructions
The patch should never be cut, as this can alter the dose delivery. It should also be kept away from direct heat sources like heating pads or saunas.
If a patch becomes loose or falls off, you should try to press it back into place. If it does not stick, a new patch should be applied for the remainder of the 24-hour period.
Making an Informed Decision
When evaluating transdermal selegiline, it is essential to align the treatment with the specific clinical goal.
- If your primary focus is finding a treatment with proven benefit over placebo: Transdermal selegiline meets this standard, showing a modest but clear advantage in clinical trials for both initial treatment and relapse prevention.
- If you are looking for a well-established first-line therapy: Its role is less certain, as it has not been directly tested against more common options like SSRIs, making its relative effectiveness unknown.
- If you are considering this for a patient under 25: Extreme caution is warranted, and it is not recommended for children due to the documented risk of increased suicidal thoughts.
Understanding these specific effectiveness rates and limitations is the first step toward a well-informed discussion with your healthcare provider.
Summary Table:
| Aspect | Key Information |
|---|---|
| Clinical Response Rate | 33-40% (vs. 22-30% for placebo) |
| Relapse Prevention | More effective than placebo over one year |
| Comparative Data | Lacks head-to-head trials vs. SSRIs/SNRIs |
| Standard Dosing | Start at 6 mg/24h, up to 12 mg/24h |
| Key Limitation | Not recommended for patients under 18 |
Looking for a reliable manufacturing partner for your transdermal patch product?
At Enokon, we are a bulk manufacturer of reliable transdermal patches and pain plasters for healthcare and pharmaceutical distributors and brands. If you are developing a specialized treatment like a selegiline patch, our technical expertise is at your service for custom R&D and development to ensure high-quality, effective delivery.
Contact our team today to discuss your project and benefit from our manufacturing excellence.
Visual Guide
Related Products
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Herbal Eye Protection Patch Eye Patch
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Heating Pain Relief Patches for Menstrual Cramps
- Icy Hot Menthol Medicine Pain Relief Patch
People Also Ask
- How do Deep Heat Pain Relief Patches provide pain relief? Discover the Drug-Free Mechanism
- Are heat patches safe for all body parts? Key Safety Zones and No-Go Areas Explained
- How does capsaicin work in the medicated heat patch? The Science Behind Pain Relief
- What are the common side effects of using the medicated heat patch? Understanding Risks & Safe Use
- What did the UK Million Women Study find regarding transdermal versus oral hormone therapy? A Safer Choice for Gallbladder Health














