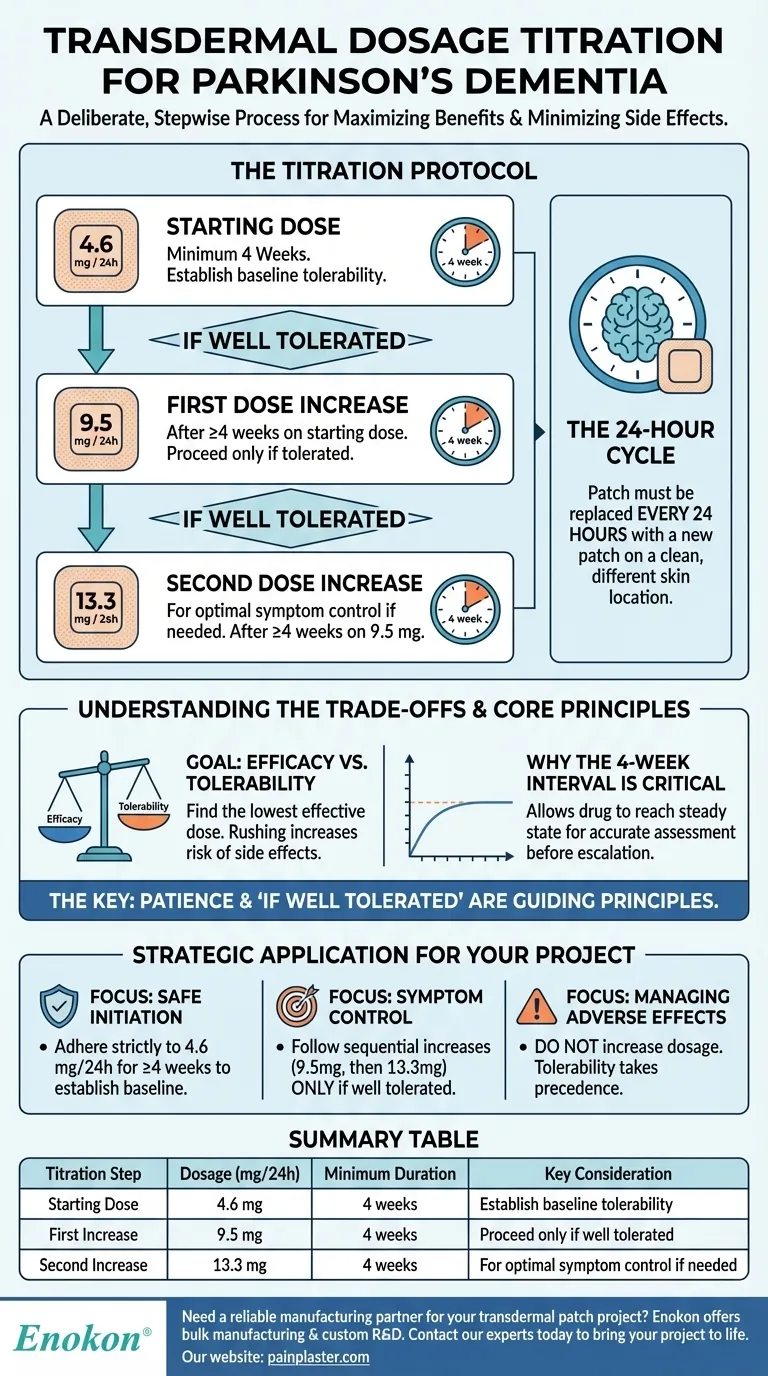
Titrating the transdermal dosage for Parkinson's dementia is a deliberate, stepwise process designed to maximize benefits while minimizing side effects. The treatment protocol begins with a starting dose of 4.6 mg applied every 24 hours. After a minimum of four weeks, and only if the initial dose is well tolerated, it may be increased to 9.5 mg every 24 hours. A further increase to 13.3 mg every 24 hours can be considered after another four weeks if needed.
The core principle of transdermal titration is patience. The slow, methodical dose increases, spaced at least four weeks apart, are critical for allowing the patient's body to acclimate and for accurately balancing therapeutic efficacy against individual tolerability.
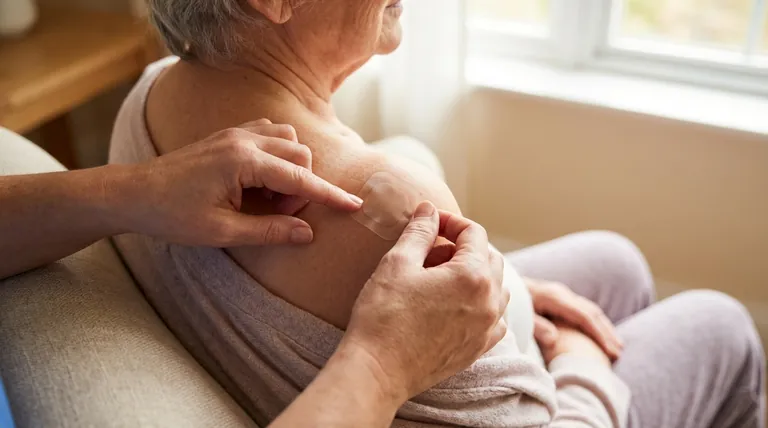
The Titration Protocol Explained
The structured approach to dose increases is the standard of care for initiating this therapy. Each step is contingent on the patient's response to the previous one.
The Starting Dose
Treatment must always begin with the 4.6 mg per 24 hours patch. This initial low dose serves as a crucial period to establish a baseline of tolerability and allow the body to adjust to the medication.
The First Dose Increase
After a minimum of four weeks on the starting dose, the dosage may be increased to 9.5 mg per 24 hours. This increase is only appropriate if the 4.6 mg dose has been well tolerated by the patient.
The Second Dose Increase
If a higher dose is clinically necessary for symptom management, it can be increased again to 13.3 mg per 24 hours. This step should only be taken after the patient has been on the 9.5 mg dose for at least another four weeks with continued good tolerability.
The 24-Hour Cycle
It is essential to remember that the patch must be replaced with a new one every 24 hours. The old patch should be removed before a new one is applied to a different, clean skin location.
Understanding the Trade-offs
The slow titration schedule is not arbitrary; it is a fundamental safety measure designed to navigate the delicate balance between managing symptoms and preventing adverse effects.
The Goal: Efficacy vs. Tolerability
The primary goal is to find the lowest effective dose for each individual. Rushing the titration process significantly increases the risk of side effects, which can lead to discomfort and potential discontinuation of the therapy.
Why the 4-Week Interval is Critical
The four-week waiting period between dose adjustments is essential. It provides enough time for the drug to reach a steady state in the body and for a clinician to accurately assess both the therapeutic benefits and any potential negative reactions before making a decision to escalate the dose.
The Importance of "If Well Tolerated"
The phrase "if well tolerated" is the guiding principle of this entire process. Before any dose increase, a careful assessment must confirm the absence of significant adverse effects. If the patient experiences issues, the dose should not be increased.
How to Apply This to Your Project
Your strategy should be dictated by the patient's individual response and the overarching clinical goals.
- If your primary focus is initiating treatment safely: Adhere strictly to the 4.6 mg/24 hours starting dose for a minimum of four weeks to establish a clear baseline of patient tolerance.
- If your primary focus is improving symptom control: Follow the sequential, four-week interval increases to 9.5 mg and then 13.3 mg, proceeding to the next step only after confirming good tolerability.
- If your primary focus is managing adverse effects: Do not increase the dosage. Patient tolerability always takes precedence over achieving a higher dose on a fixed schedule.
A patient, methodical approach to titration is the key to successfully managing Parkinson's dementia with this transdermal therapy.
Summary Table:
| Titration Step | Dosage (mg/24h) | Minimum Duration | Key Consideration |
|---|---|---|---|
| Starting Dose | 4.6 mg | 4 weeks | Establish baseline tolerability |
| First Increase | 9.5 mg | 4 weeks | Proceed only if well tolerated |
| Second Increase | 13.3 mg | 4 weeks | For optimal symptom control if needed |
Need a reliable manufacturing partner for your transdermal patch project?
At Enokon, we are a bulk manufacturer of reliable transdermal patches and pain plasters for healthcare and pharmaceutical distributors and brands. Our technical expertise supports custom R&D and development, ensuring your product, like a Parkinson's dementia patch, meets precise dosage and delivery requirements.
Let's discuss your specific needs. Contact our experts today to explore how we can bring your project to life.
Visual Guide
Related Products
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Capsaicin Chili Medicated Pain Relief Patches
- Heating Pain Relief Patches for Menstrual Cramps
- Herbal Eye Protection Patch Eye Patch
- Icy Hot Menthol Medicine Pain Relief Patch
People Also Ask
- Can heat patches be used for fresh injuries? Avoid This Common Mistake for Faster Recovery
- What types of pain can the Deep Heat Pain Relief Back Patch be used for? Targeted Relief for Muscles & Joints
- What are the key features of the Deep Heat Pain Relief Back Patch? Get Up to 16 Hours of Drug-Free Relief
- How does the Deep Heat Back Patch work? A Drug-Free Solution for Targeted Pain Relief
- How does capsaicin work in the medicated heat patch? The Science Behind Pain Relief















