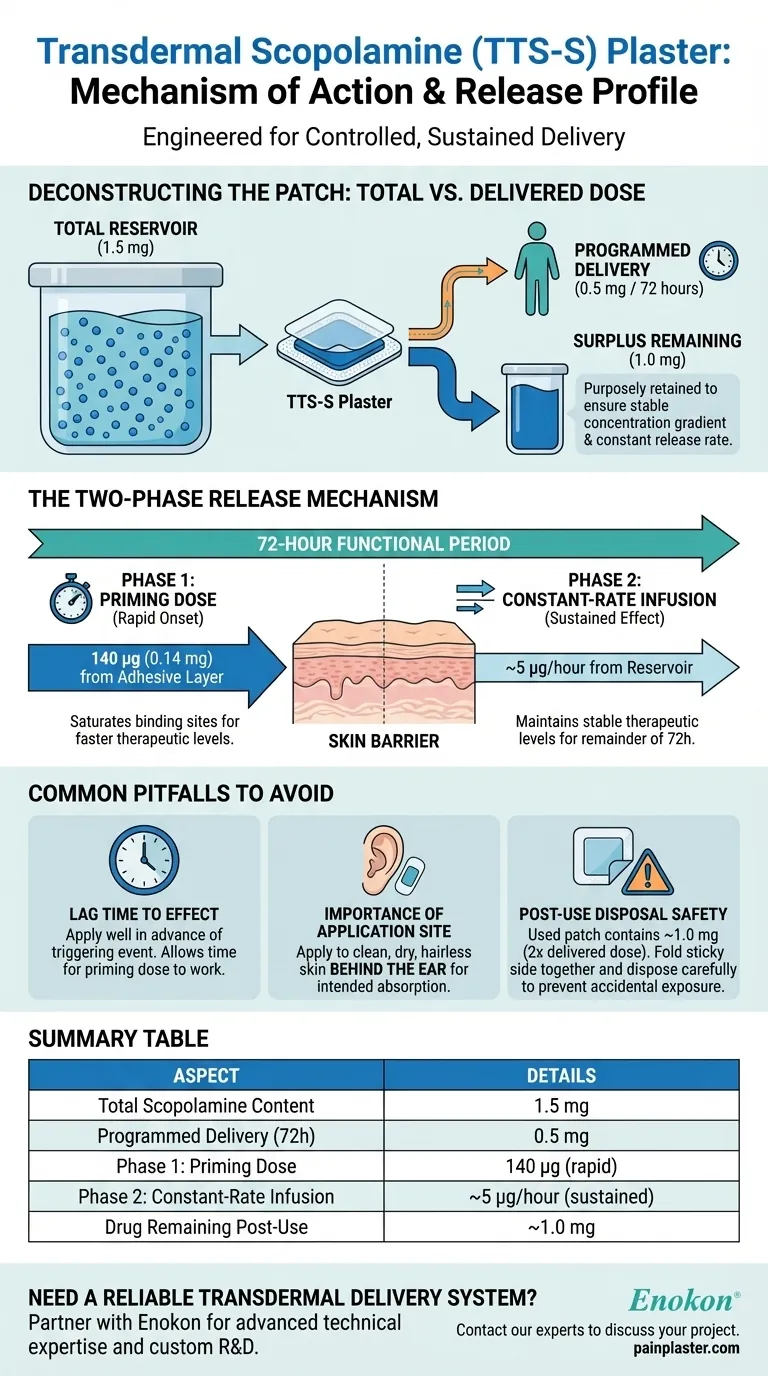
The transdermal scopolamine (TTS-S) plaster contains a total reservoir of 1.5 mg of the drug. It is engineered to deliver a much smaller, controlled dose of 0.5 mg over a three-day (72-hour) period through a sophisticated two-phase release mechanism.
The patch is not a simple reservoir but an engineered delivery system. It uses an initial priming dose for rapid onset, followed by a constant-rate release for sustained effect, intentionally retaining a large surplus of the drug to ensure this rate remains stable.

Deconstructing the Patch: Total Content vs. Delivered Dose
To understand the patch's function, it's crucial to distinguish between the total amount of scopolamine it holds and the amount it is designed to deliver to the user.
The Total Reservoir: 1.5 mg
Each plaster contains a total of 1.5 mg of scopolamine. This entire amount is not meant to be absorbed.
The Programmed Delivery: 0.5 mg
The system is precisely calibrated to deliver only 0.5 mg of scopolamine over its 3-day functional lifespan. This means a significant amount of the drug remains in the patch upon removal.
The Purpose of the Surplus
The large excess of scopolamine (1.0 mg remaining) is a key design feature. It ensures a stable concentration gradient, which is the driving force for the drug to move from the patch into the skin. This surplus guarantees that the release rate remains constant, even as the patch is partially depleted.
The Two-Phase Release Mechanism
The delivery of the 0.5 mg dose is not linear. It occurs in two distinct, engineered phases to optimize the therapeutic effect.
Phase 1: The Priming Dose (140 µg)
An initial dose of 140 micrograms (0.14 mg) is incorporated directly into the adhesive layer of the patch. This portion is delivered rapidly upon application.
The Rationale: Overcoming the Skin Barrier
This priming dose serves a critical function: to quickly saturate the binding sites within the skin. By overcoming this initial biological barrier, the drug can reach the bloodstream and achieve stable, therapeutic levels much faster than a simple reservoir-only patch could.
Phase 2: Constant-Rate Infusion (~5 µg/hour)
After the initial priming dose is delivered, the patch transitions to its main function. The remaining scopolamine is released from the reservoir at a steady, controlled rate of approximately 5 micrograms per hour for the rest of the 72-hour period.
Common Pitfalls to Avoid
Understanding the design of the TTS-S patch helps prevent common errors in its use and interpretation.
Lag Time to Effect
Even with a priming dose, it takes time for the drug to reach effective concentrations in the body. Patients should apply the patch several hours before a triggering event (like travel) for it to be effective.
Importance of Application Site
The specified absorption rates are based on application to the intended area (typically the clean, dry, hairless skin behind the ear). Placing the patch elsewhere can alter the rate of absorption and its effectiveness.
The Remaining Drug Post-Use
A used patch still contains approximately 1.0 mg of scopolamine—twice the amount delivered to the user. It must be folded in half with the sticky side together and disposed of carefully to prevent accidental exposure to children or pets.
Making the Right Choice for Your Goal
Your clinical application of this knowledge depends on your primary objective.
- If your primary focus is rapid onset of action: Advise patients to apply the patch well in advance, as the priming dose is designed to accelerate, not eliminate, the time to reach steady-state blood levels.
- If your primary focus is sustained effect: You can be confident the system's constant-rate delivery of 5 µg/hour is designed for reliable, long-term prevention of motion sickness over 72 hours.
- If your primary focus is patient safety: Emphasize the critical importance of proper disposal, as a used patch remains a potent source of active medication.
By understanding its two-phase design, you can leverage the transdermal scopolamine patch for its intended safe and consistent therapeutic outcomes.
Summary Table:
| Aspect | Details |
|---|---|
| Total Scopolamine Content | 1.5 mg |
| Programmed Delivery (over 72 hours) | 0.5 mg |
| Phase 1: Priming Dose | 140 µg (rapid delivery) |
| Phase 2: Constant-Rate Infusion | ~5 µg/hour (sustained delivery) |
| Drug Remaining Post-Use | ~1.0 mg |
Need a reliable transdermal delivery system for your pharmaceutical product?
As Enokon, a bulk manufacturer of reliable transdermal patches and pain plasters, we provide our partners—healthcare and pharma distributors and brands—with advanced technical expertise for custom R&D and development. Let us help you engineer a precise and effective transdermal solution.
Contact our experts today to discuss your project requirements.
Visual Guide
Related Products
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Heating Pain Relief Patches for Menstrual Cramps
- Natural Herbal Wormwood Patch Pain Plaster
- Far Infrared Pain Patch Relief Pain Reliever for Back
- Heat Relief Capsicum Patch for Lower Back Pain Relief
People Also Ask
- How does the Deep Heat Back Patch work? A Drug-Free Solution for Targeted Pain Relief
- How does capsaicin work in the medicated heat patch? The Science Behind Pain Relief
- What are the key features of the Deep Heat Pain Relief Back Patch? Get Up to 16 Hours of Drug-Free Relief
- Are heat patches safe for all body parts? Key Safety Zones and No-Go Areas Explained
- What did the UK Million Women Study find regarding transdermal versus oral hormone therapy? A Safer Choice for Gallbladder Health















