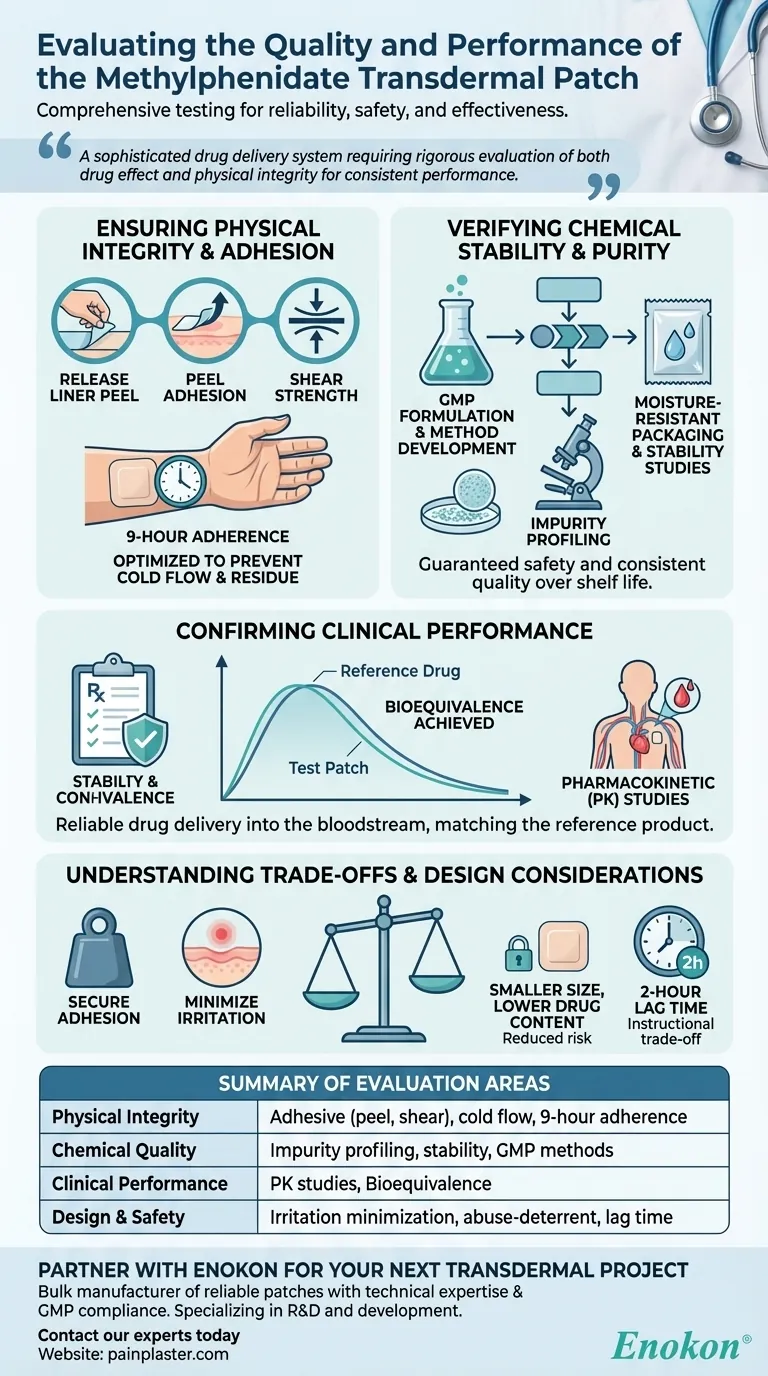
To ensure its quality and performance, the methylphenidate transdermal patch was evaluated through a comprehensive series of tests covering its physical properties, chemical stability, and clinical effectiveness. Key assessments included adhesive performance, stability studies, impurity profiling, and clinical pharmacokinetic studies to confirm it delivers the medication reliably and is bioequivalent to the reference drug.
A transdermal patch is more than just a sticker with medicine; it's a sophisticated drug delivery system. Its evaluation, therefore, must scrutinize not only the drug's effect but also the physical integrity of the patch itself to guarantee safe, consistent, and predictable performance from application to removal.
Ensuring Physical Integrity and Adhesion
A patch is useless if it doesn't stick properly. The physical tests are designed to ensure the patch remains securely in place for its intended duration while being comfortable for the user.
Optimizing Adhesive Properties
The core adhesive qualities were measured using standardized tests. This includes release liner peel (how easily it comes off its backing), peel adhesion (how well it sticks to skin), and shear (its ability to resist sliding forces from movement).
Preventing Cold Flow and Residue
"Cold flow" is when the adhesive spreads beyond the patch's edges, which can lead to messy residue and inaccurate dosing. The formulation was optimized to prevent this, ensuring a clean application and removal.
Guaranteeing Adherence
The ultimate goal is for the patch to adhere to the skin for the full prescribed time, typically nine hours. The combination of these physical tests confirms the patch can withstand real-world conditions like friction from clothing and patient movement.
Verifying Chemical Stability and Purity
The patch must not only deliver the drug but also protect it from degradation. This requires rigorous chemical analysis and thoughtful design.
Formulation and Method Development
The process began with developing a stable drug formulation and the analytical methods needed to measure it accurately. These procedures, established under Good Manufacturing Practices (GMP), are the foundation for consistent quality control.
Assessing the Impurity Profile
Every potential chemical impurity was identified and quantified. This assessment is critical to ensure that no harmful byproducts are formed during manufacturing or storage, guaranteeing patient safety.
Designing for Long-Term Stability
To prevent chemical degradation from environmental factors, the patch was designed with moisture-resistant packaging. Stability studies confirmed the product remains safe and effective throughout its shelf life.
Confirming Clinical Performance
Once the physical and chemical properties are verified, the patch must be proven to work effectively in the human body.
The Role of Pharmacokinetic Studies
Clinical pharmacokinetic (PK) studies are essential. These trials measure the rate and extent to which methylphenidate is absorbed into the bloodstream from the patch.
Achieving Bioequivalence
The primary goal of the PK studies was to demonstrate bioequivalence. This means the patch delivers the same amount of active ingredient into the bloodstream over the same period as the original, approved drug, ensuring equivalent therapeutic effects.
Understanding the Trade-offs and Design Considerations
Every drug delivery system involves balancing competing factors. The patch's design reflects deliberate choices made to optimize safety and effectiveness.
Balancing Adhesion with Skin Irritation
An overly aggressive adhesive can cause skin irritation or damage upon removal. The design balances secure adhesion with being gentle on the skin, which is why rotating the application site daily between hips is a key instruction.
Minimizing Risk of Exposure and Abuse
Compared to other options, the patch was intentionally designed with a smaller size and lower total drug content. This reduces the risk of accidental exposure for family members and makes it a less likely target for potential abuse.
The 2-Hour Lag Time
The instruction to apply the patch two hours before an effect is needed highlights a key trade-off of transdermal delivery. It takes time for the medication to travel through the skin layers and into the bloodstream, a contrast to the faster onset of an oral pill.
How This Evaluation Ensures Reliable Treatment
The multi-faceted testing protocol is designed to answer the fundamental questions for any medical product: is it safe, is it effective, and is it reliable?
- If your primary focus is consistent dosing: The critical evaluations are the pharmacokinetic studies confirming bioequivalence and the adhesive tests ensuring the patch stays on for the full nine hours.
- If your primary focus is patient safety: The key assessments are the impurity profiling, the use of moisture-resistant packaging, and the design choice for a smaller patch with lower drug content.
- If your primary focus is user compliance: The evaluation of adhesive properties to prevent residue and the clinical guidance to rotate sites to minimize irritation are most important.
Ultimately, this rigorous evaluation ensures the methylphenidate transdermal patch is a dependable and well-characterized medical device.
Summary Table:
| Evaluation Area | Key Tests & Focus |
|---|---|
| Physical Integrity | Adhesive performance (peel, shear), cold flow prevention, 9-hour adherence |
| Chemical Quality | Impurity profiling, stability studies, GMP method development |
| Clinical Performance | Pharmacokinetic (PK) studies, bioequivalence to reference drug |
| Design & Safety | Skin irritation minimization, abuse-deterrent features, lag time management |
Partner with Enokon for Your Next Transdermal Patch Project
As a bulk manufacturer of reliable transdermal patches and pain plasters, Enokon provides the technical expertise and GMP-compliant manufacturing essential for success. We specialize in custom R&D and development for healthcare and pharmaceutical distributors and brands, ensuring your product meets the highest standards for adhesion, stability, and clinical performance.
Contact our experts today to discuss how we can support your product development from formulation to final packaging.
Visual Guide
Related Products
- Icy Hot Menthol Medicine Pain Relief Patch
- Mugwort Wormwood Pain Relief Patch for Neck Pain
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Capsaicin Chili Medicated Pain Relief Patches
- Menthol Gel Pain Relief Patch
People Also Ask
- How does menthol in the patch work to relieve pain? Discover the Science Behind Fast-Acting Relief
- Can cooling patches be used on newborns? Safe Fever Relief for Infants
- How should a menthol patch be applied? Follow These Steps for Safe & Effective Pain Relief
- What are common side effects of menthol patch? Key Risks & Safety Tips
- What are the important warnings for using menthol topical? Safety Tips for Effective Pain Relief














