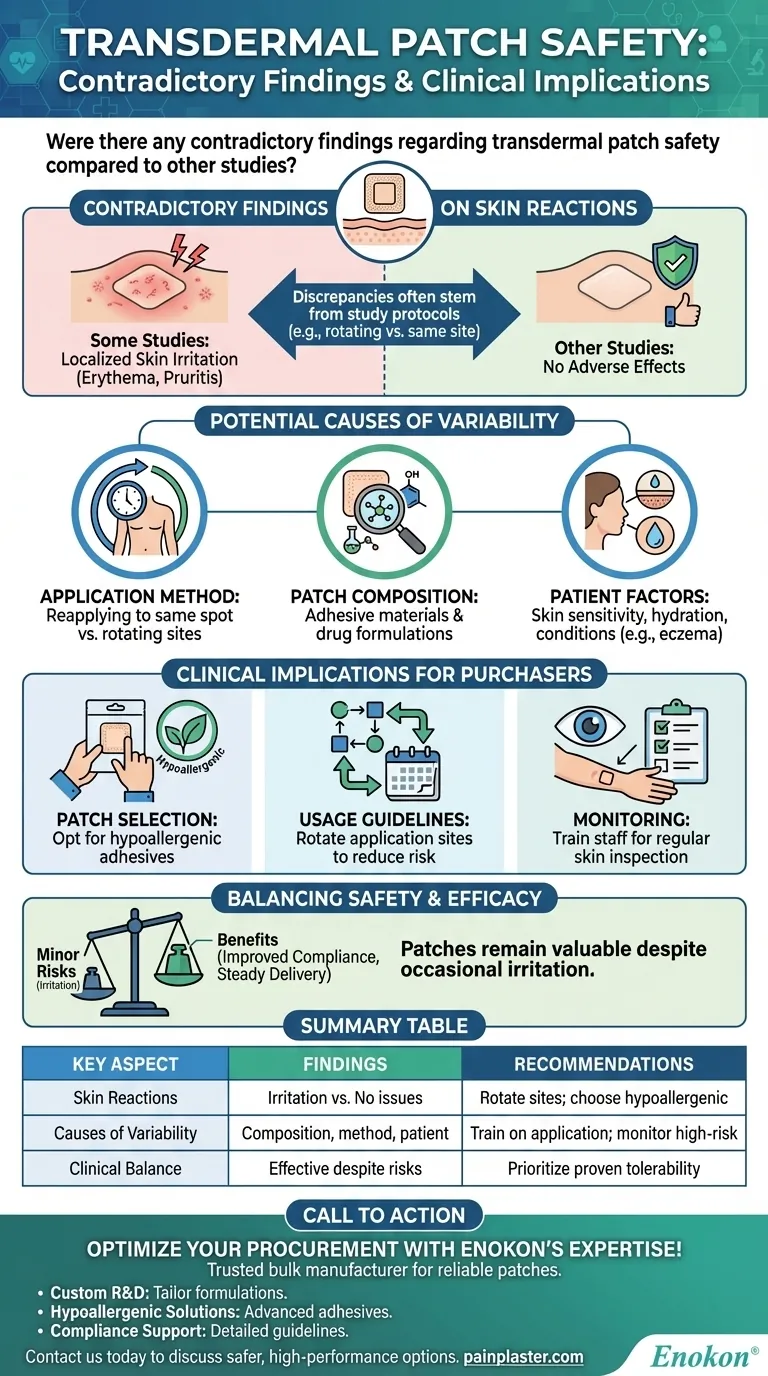
Transdermal patches are generally considered safe and effective for drug delivery, but some contradictory findings exist regarding skin reactions. While certain studies report localized skin irritation like erythematous rash or pruritis at the application site, others found no such effects. These discrepancies may stem from differences in study protocols, such as rotating application sites between doses versus reapplying to the same location. The variability highlights the importance of proper patch application techniques and monitoring for adverse reactions in clinical practice.
Key Points Explained:
-
Contradictory Findings on Skin Reactions
- Some studies report localized skin irritation (erythema, pruritis) with transdermal patch use, while others observed no adverse effects.
- Example: One study found no irritation when patches were applied to different sites successively, suggesting rotation minimizes skin reactions.
-
Potential Causes of Variability
- Application Method: Reapplying patches to the same spot may increase irritation risk due to prolonged skin exposure.
- Patch Composition: Differences in adhesive materials or drug formulations across products could affect tolerability.
- Patient Factors: Skin sensitivity, hydration, and pre-existing conditions (e.g., eczema) may influence reactions.
-
Clinical Implications for Purchasers
- Patch Selection: Opt for brands with hypoallergenic adhesives if skin irritation is a concern.
- Usage Guidelines: Ensure protocols include rotating application sites to reduce adverse events.
- Monitoring: Train staff to inspect skin regularly, especially in long-term therapy.
-
Balancing Safety and Efficacy
- Despite occasional irritation, transdermal patches remain valuable for non-invasive, steady drug delivery.
- Weighing benefits (e.g., improved compliance) against minor risks is key for healthcare procurement decisions.
Have you considered how patch material innovations could further reduce these inconsistencies? Advances in silicone-based adhesives, for instance, are already enhancing patient comfort while maintaining drug stability—a quiet but impactful evolution in medical technology.
Summary Table:
| Key Aspect | Findings | Recommendations |
|---|---|---|
| Skin Reactions | Some studies report irritation (erythema, pruritis); others show no issues. | Rotate application sites; choose hypoallergenic adhesives. |
| Causes of Variability | Patch composition, application method, and patient factors influence risks. | Train staff on proper application; monitor high-risk patients. |
| Clinical Balance | Patches remain effective despite minor risks. | Prioritize brands with proven tolerability and stable drug delivery. |
Optimize your transdermal patch procurement with Enokon’s expertise!
As a trusted bulk manufacturer of reliable transdermal patches and pain plasters, we help healthcare distributors and brands mitigate safety concerns through:
- Custom R&D: Tailor formulations to minimize skin irritation.
- Hypoallergenic Solutions: Advanced adhesives for sensitive patients.
- Compliance Support: Detailed usage guidelines for your clinical teams.
Contact us today to discuss safer, high-performance patch options for your inventory.
Visual Guide
Related Products
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Heating Pain Relief Patches for Menstrual Cramps
- Herbal Eye Protection Patch Eye Patch
- Menthol Gel Pain Relief Patch
- Icy Hot Menthol Medicine Pain Relief Patch
People Also Ask
- What did the UK Million Women Study find regarding transdermal versus oral hormone therapy? A Safer Choice for Gallbladder Health
- How does capsaicin work in the medicated heat patch? The Science Behind Pain Relief
- How quickly does the Deep Heat Pain Relief Back Patch activate and how long does it provide warmth? Get 16-Hour Relief
- What are the common side effects of using the medicated heat patch? Understanding Risks & Safe Use
- What types of pain can the Deep Heat Pain Relief Back Patch be used for? Targeted Relief for Muscles & Joints














