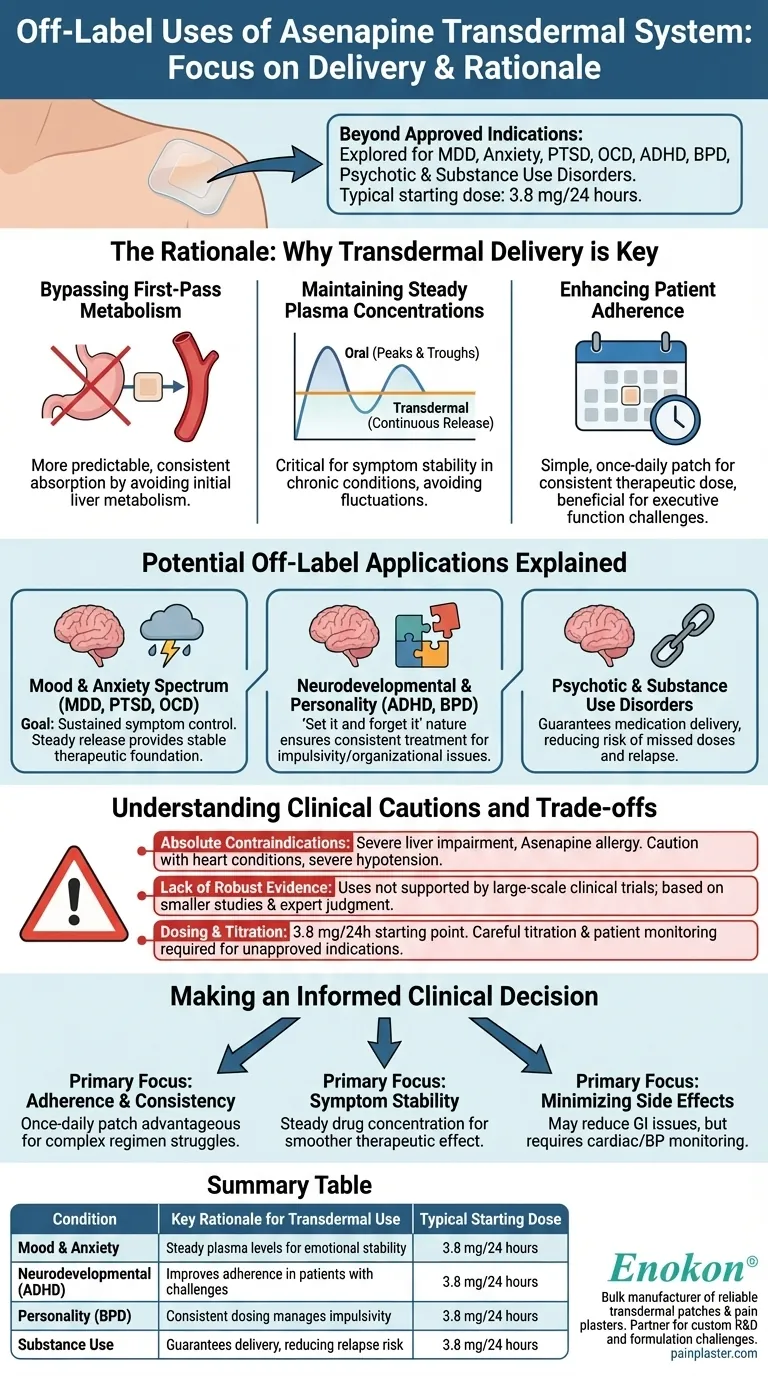
Beyond its approved indications, the Asenapine transdermal system is used off-label for a range of psychiatric conditions, including major depressive disorder (MDD), anxiety disorders, PTSD, OCD, ADHD, borderline personality disorder (BPD), other psychotic disorders, and substance use disorders. The typical starting dose for these applications is 3.8 mg/24 hours, leveraging the patch's unique delivery mechanism to address complex symptoms.
The exploration of Asenapine for off-label use is driven less by the drug itself and more by the distinct advantages of its transdermal delivery system. Clinicians are leveraging the patch to provide steady medication levels, improve patient adherence, and potentially reduce certain side effects for challenging-to-treat conditions.

The Rationale: Why Transdermal Delivery is Key
Understanding the off-label application of Asenapine requires focusing on the delivery system. The decision to use a patch over an oral medication is often a strategic choice to optimize treatment outcomes.
Bypassing First-Pass Metabolism
Oral medications must first pass through the digestive system and liver, where a significant portion can be metabolized before reaching the bloodstream. The Asenapine patch bypasses this "first-pass effect," allowing for more predictable and consistent absorption of the drug.
Maintaining Steady Plasma Concentrations
Unlike the peaks and troughs associated with oral dosing, a transdermal system provides a continuous and controlled release of medication. This steady-state concentration can be critical for maintaining symptom stability in chronic psychiatric conditions, avoiding breakthrough symptoms or side effects linked to fluctuating drug levels.
Enhancing Patient Adherence
A simple, once-daily patch can significantly improve treatment adherence compared to multi-dose oral regimens. This is particularly beneficial for patients with conditions that may impact executive function or memory, ensuring they receive a consistent therapeutic dose without the burden of remembering to take pills.
Potential Off-Label Applications Explained
The benefits of transdermal delivery provide a clear rationale for its consideration across several categories of psychiatric illness.
Mood and Anxiety Spectrum Disorders (MDD, PTSD, OCD)
For these conditions, the primary goal is often sustained symptom control. The steady release from the patch may help smooth out emotional and anxious fluctuations more effectively than oral medications, providing a more stable therapeutic foundation.
Neurodevelopmental and Personality Disorders (ADHD, BPD)
In conditions like ADHD or BPD, impulsivity and organizational challenges can make adherence to oral medication difficult. The "set it and forget it" nature of a daily patch helps ensure consistent treatment, which is fundamental to managing symptoms.
Psychotic and Substance Use Disorders
Consistency is paramount in preventing relapse in severe psychotic disorders and substance use disorders. The transdermal system guarantees medication delivery as long as it is worn, reducing the risk associated with intentionally or unintentionally missed doses.
Understanding the Clinical Cautions and Trade-offs
While the potential benefits are clear, off-label use requires careful consideration of the risks and limitations. This approach is not suitable for every patient.
Absolute Contraindications
The Asenapine transdermal system should not be used in patients with severe liver impairment or a known allergic reaction to Asenapine. It requires extreme caution in those with pre-existing heart conditions, such as arrhythmias, or severe hypotension (low blood pressure).
The Lack of Robust Evidence
It is critical to recognize that "off-label" means these uses are not supported by the large-scale, rigorous clinical trials required for official approval. The evidence is often based on smaller studies, case reports, or clinical experience, requiring careful expert judgment.
Dosing and Titration
The system is available in three strengths: 3.8 mg/24 hours, 5.7 mg/24 hours, and 7.6 mg/24 hours. While 3.8 mg is a common starting point, titrating to the optimal dose for an unapproved indication requires careful patient monitoring and a clear clinical rationale.
Making an Informed Clinical Decision
When considering the Asenapine transdermal system off-label, the choice must be guided by the specific goals of treatment for the individual patient.
- If your primary focus is treatment adherence and consistency: The transdermal system's once-daily application offers a clear advantage for patients who struggle with complex oral medication regimens.
- If your primary focus is symptom stability in chronic conditions: The steady drug concentration provided by the patch may offer a smoother therapeutic effect compared to the peaks and troughs of oral dosing.
- If your primary focus is minimizing certain side effects: This system may reduce gastrointestinal issues by bypassing the digestive system, but careful monitoring for cardiac and blood pressure effects remains essential.
Ultimately, the decision to use the Asenapine patch off-label is a clinical judgment that balances the unique pharmacokinetic benefits of transdermal delivery against the individual patient's needs and risk factors.
Summary Table:
| Off-Label Condition | Key Rationale for Transdermal Use | Typical Starting Dose |
|---|---|---|
| Mood & Anxiety Disorders (MDD, PTSD, OCD) | Steady plasma levels for emotional stability | 3.8 mg/24 hours |
| Neurodevelopmental Disorders (ADHD) | Improves adherence in patients with organizational challenges | 3.8 mg/24 hours |
| Personality Disorders (BPD) | Consistent dosing manages impulsivity and improves outcomes | 3.8 mg/24 hours |
| Substance Use Disorders | Guarantees medication delivery, reducing relapse risk from missed doses | 3.8 mg/24 hours |
Need a reliable transdermal delivery solution for your psychiatric medications?
As Enokon, a bulk manufacturer of reliable transdermal patches and pain plasters, we partner with healthcare and pharma distributors and brands to overcome formulation challenges. Our technical expertise in custom R&D and development can help you create effective, patient-friendly transdermal systems that improve adherence and therapeutic outcomes.
Contact our experts today to discuss your custom transdermal project.
Visual Guide
Related Products
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Capsaicin Chili Medicated Pain Relief Patches
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Heating Pain Relief Patches for Menstrual Cramps
- Asthma Cough and Pain Relief Patch for Adults and Kids
People Also Ask
- How does the Deep Heat Back Patch work? A Drug-Free Solution for Targeted Pain Relief
- What are the common side effects of using the medicated heat patch? Understanding Risks & Safe Use
- What are the key features of the Deep Heat Pain Relief Back Patch? Get Up to 16 Hours of Drug-Free Relief
- How does capsaicin work in the medicated heat patch? The Science Behind Pain Relief
- What types of pain can the Deep Heat Pain Relief Back Patch be used for? Targeted Relief for Muscles & Joints















