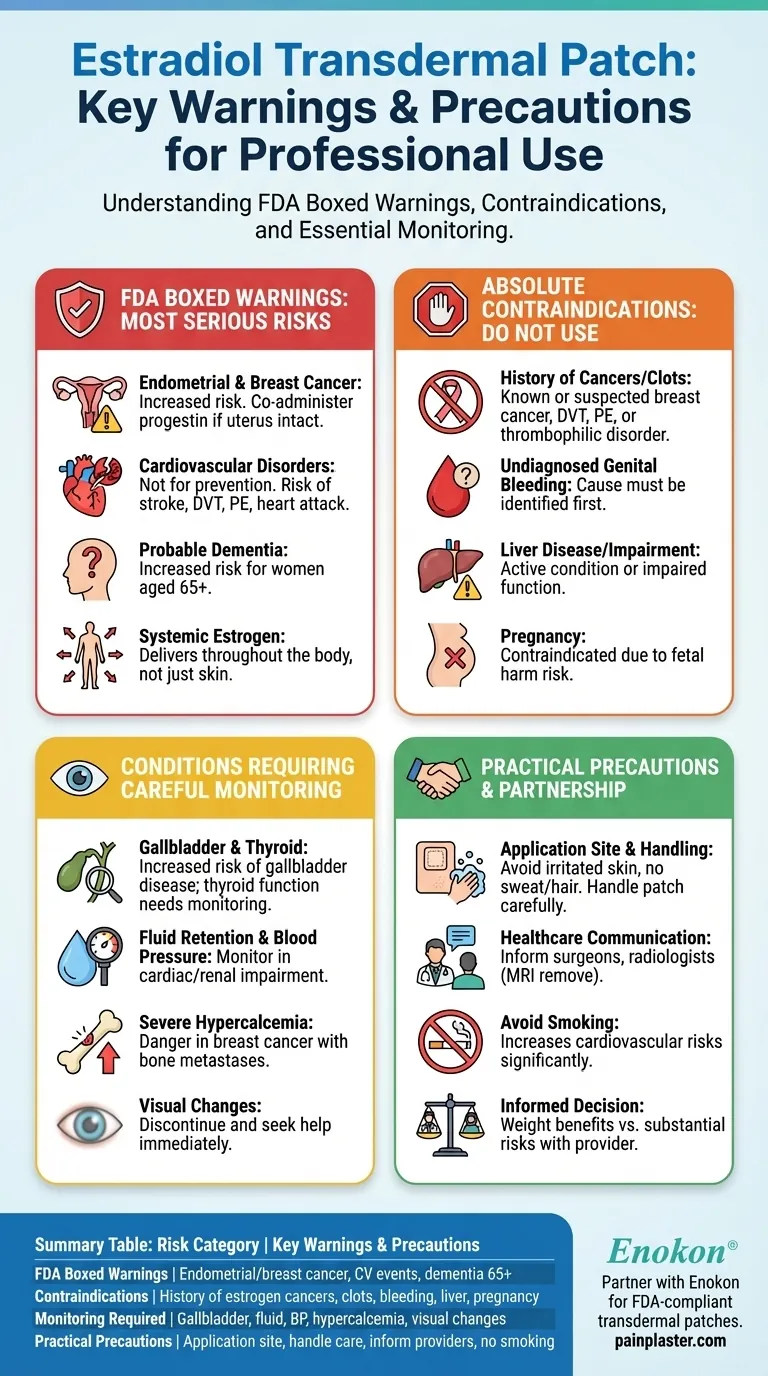
The estradiol transdermal patch, a form of systemic hormone therapy, carries significant warnings and precautions that must be understood. The most serious risks, highlighted in FDA boxed warnings, include an increased risk of endometrial and breast cancer, cardiovascular disorders like stroke and heart attack, and probable dementia in women over 65.
The core issue to understand is that the estradiol patch is not a localized skin treatment; it delivers estrogen systemically throughout your body. Therefore, its use requires a careful evaluation of your personal and family medical history against a clear set of established risks.

The Four FDA Boxed Warnings: Understanding the Most Serious Risks
The U.S. Food and Drug Administration (FDA) mandates "boxed warnings" for medications with the most serious potential side effects. For the estradiol patch, these center on four critical areas.
Endometrial and Breast Cancer
Using estrogen alone significantly increases the risk of endometrial cancer (cancer of the uterine lining) in a woman with an intact uterus.
To mitigate this risk, it is essential to co-administer a progestin. Estrogen use has also been linked to an increased risk of breast cancer.
Cardiovascular Disorders
Estrogen therapy should not be used for the prevention of cardiovascular disease.
Studies show an increased risk of serious cardiovascular events, including stroke, deep vein thrombosis (DVT), pulmonary embolism (PE), and myocardial infarction (MI) in postmenopausal women using this therapy.
Probable Dementia
For postmenopausal women aged 65 or older, using estrogen-alone therapy or estrogen-plus-progestin therapy increases the risk of developing probable dementia.
When the Estradiol Patch is Absolutely Contraindicated
Certain pre-existing conditions make using the estradiol patch unsafe. These are known as contraindications, and their presence means you should not use this medication.
History of Cancers or Blood Clots
Do not use this patch if you have a known or suspected history of breast cancer or any other estrogen-dependent neoplasm.
It is also contraindicated in anyone with a current or past history of DVT or PE, or a known thrombophilic disorder (like Protein C, Protein S, or antithrombin deficiency).
Undiagnosed Genital Bleeding
Any instance of undiagnosed and abnormal genital bleeding is a firm contraindication. The cause must be identified before considering hormone therapy.
Liver Disease or Impairment
You should not use the estradiol patch if you have active liver disease or impaired liver function.
Known or Suspected Pregnancy
This medication is contraindicated for use during pregnancy as it can cause fetal harm.
Key Conditions Requiring Careful Monitoring
Even if the patch is not strictly contraindicated, certain conditions require close and ongoing monitoring by your physician while you are using it.
Gallbladder and Thyroid Function
There is a known increased risk of gallbladder disease requiring surgery in women receiving postmenopausal estrogens. Your doctor may also need to monitor your thyroid function.
Fluid Retention and Blood Pressure
Estrogens can cause fluid retention. If you have a condition that could be worsened by this, such as cardiac or renal impairment, you require careful observation. Blood pressure should also be monitored regularly.
Severe Hypercalcemia
In patients with breast cancer and bone metastases, estrogen administration can lead to severe hypercalcemia (dangerously high calcium levels). If this occurs, the patch must be discontinued.
Visual Changes
Discontinue the patch immediately and seek medical attention if you experience sudden partial or complete loss of vision, or the sudden onset of double vision or migraine.
Practical Precautions and Common Pitfalls
Safe use extends beyond medical monitoring to proper daily handling and communication.
Choosing the Right Application Site
Avoid placing the patch on skin that has cuts, creases, or irritation. Do not apply it to areas that get sweaty, have a lot of hair, or will be rubbed by clothing, like under a belt. Wait at least three days after shaving an area before applying a patch there.
Handling the Patch Safely
When opening the pouch, be careful not to tear the patch itself. Avoid touching the adhesive side, but if you do, wash your hands thoroughly with soap and water.
Communicating with All Healthcare Providers
It is critical to inform all medical personnel, including surgeons and radiologists, that you are using this patch. Its use may need to be discontinued before a scheduled surgery, and the patch must be removed before an MRI.
Avoiding Smoking
Smoking significantly increases the risk of serious cardiovascular side effects, including blood clots, stroke, and heart attack. You should avoid smoking while using the estradiol patch.
Making an Informed Decision with Your Doctor
The decision to use an estradiol patch is a significant one that involves weighing its benefits against its substantial risks. This requires an initial and ongoing conversation with your healthcare provider.
- If your primary focus is managing severe menopausal symptoms: You must also address the risks, especially the absolute necessity of progestin if you have a uterus.
- If you have a personal or strong family history of blood clots or heart disease: This therapy carries elevated risks and may not be the appropriate choice for you.
- If you are over age 65: A frank discussion with your doctor about the increased risk of probable dementia is essential.
- If you experience any abnormal bleeding or visual disturbances: You must contact your doctor immediately, as these may be signs of a serious complication.
Ultimately, using the estradiol patch safely depends on a strong partnership and open communication with your healthcare provider.
Summary Table:
| Risk Category | Key Warnings & Precautions |
|---|---|
| FDA Boxed Warnings | Increased risk of endometrial/breast cancer, cardiovascular events (stroke, heart attack), dementia in women 65+ |
| Contraindications | History of estrogen-dependent cancers, blood clots, undiagnosed genital bleeding, liver disease, pregnancy |
| Monitoring Required | Gallbladder disease, fluid retention, blood pressure, hypercalcemia, visual changes |
| Practical Precautions | Avoid certain application sites, handle patch carefully, inform all healthcare providers, do not smoke |
Need a reliable, safely manufactured transdermal patch? Partner with Enokon, a bulk manufacturer of FDA-compliant transdermal patches and pain plasters for healthcare distributors and pharmaceutical brands. Our technical expertise ensures custom R&D and development tailored to your safety and efficacy requirements. Contact us today to discuss your project and benefit from our quality-driven manufacturing process.
Visual Guide
Related Products
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Capsaicin Chili Medicated Pain Relief Patches
- Heating Pain Relief Patches for Menstrual Cramps
- Far Infrared Pain Patch Relief Pain Reliever for Back
People Also Ask
- How do Deep Heat Pain Relief Patches provide pain relief? Discover the Drug-Free Mechanism
- What did the UK Million Women Study find regarding transdermal versus oral hormone therapy? A Safer Choice for Gallbladder Health
- How does capsaicin work in the medicated heat patch? The Science Behind Pain Relief
- Can heat patches be used for fresh injuries? Avoid This Common Mistake for Faster Recovery
- Are heat patches safe for all body parts? Key Safety Zones and No-Go Areas Explained














