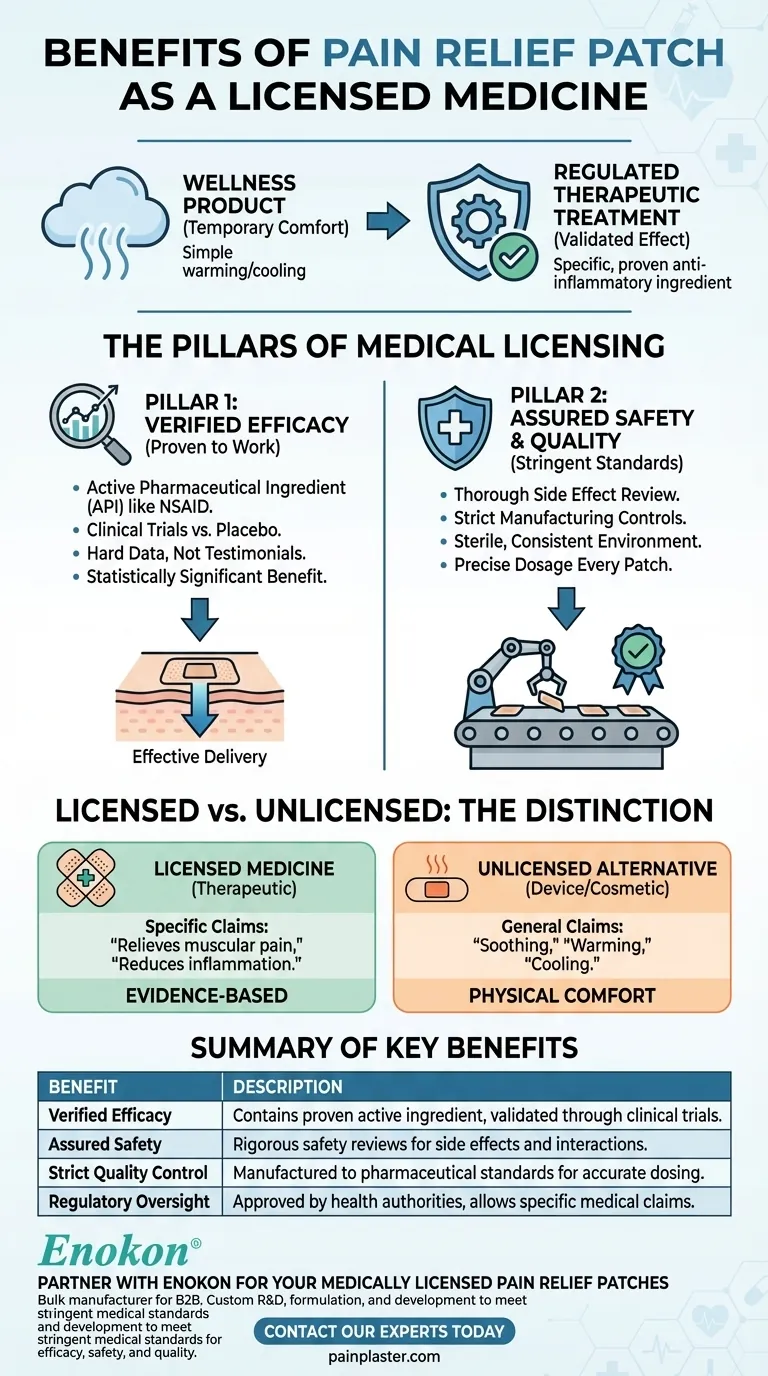
When a pain relief patch is licensed as a medicine, it signifies a fundamental shift from a simple wellness product to a regulated therapeutic treatment. This legal classification guarantees the patch contains a specific, proven anti-inflammatory ingredient, has undergone rigorous clinical trials to validate its pain-relieving claims, and has been thoroughly evaluated for safety by a regulatory authority.
A medical license is your assurance that a pain relief patch has been scientifically proven to work as advertised. It separates a tested medical treatment from products that may only offer a temporary warming or cooling sensation without a validated therapeutic effect.

The Pillars of Medical Licensing
The process of getting a product licensed as a medicine is demanding by design. It is built on two core principles: proving the product is effective for its stated purpose and ensuring it is safe for public use.
Pillar 1: Verified Efficacy
A key benefit of a licensed patch is the guarantee of efficacy, meaning it has been proven to work. This isn't based on testimonials, but on hard data.
To gain a license, the patch must contain a specific active pharmaceutical ingredient (API), such as a non-steroidal anti-inflammatory drug (NSAID). The manufacturer must then prove this ingredient is delivered effectively through the skin to relieve pain.
This proof comes from clinical trials, where the medicated patch is scientifically tested against a placebo (a patch with no medicine) to measure its actual effect on pain reduction. Only products that demonstrate a statistically significant benefit can be licensed.
Pillar 2: Assured Safety and Quality
Safety is the other cornerstone of medical licensing. A product sold as a medicine must meet stringent standards that unlicensed products are not held to.
This includes a thorough review of potential side effects, interactions with other medications, and clear instructions on proper usage to minimize risks.
Furthermore, licensing dictates strict manufacturing and quality controls. This ensures every patch contains the precise dose of medicine stated on the package and is produced in a sterile, consistent environment.
Licensed Medicine vs. Other Patches
It's crucial to understand the distinction between a licensed medicinal patch and other products on the shelf that may look similar.
The Unlicensed Alternative
Many patches are classified as "medical devices" or even cosmetics. These products typically work by physical means, such as providing heat, cold, or physical support.
While they may offer comfort, they do not contain an active medicinal ingredient. As such, they have not undergone the same level of clinical testing to prove they can treat a medical condition like pain or inflammation.
The Clarity of Claims
A licensed medicine can make specific, proven claims, such as "for the relief of muscular pain" or "reduces inflammation."
Unlicensed products are restricted to more general, non-medical claims, such as "soothing," "warming," or "cooling." This difference in language is a direct result of the regulatory evidence required.
How to Apply This to Your Choice
When selecting a pain relief product, understanding this distinction empowers you to choose the right tool for your specific goal.
- If your primary focus is proven, targeted pain relief: Choose a patch licensed as a medicine, as its effectiveness has been scientifically validated through clinical trials.
- If your primary focus is safety and controlled dosage: A licensed patch is the superior option, as it has undergone rigorous safety reviews and quality-controlled manufacturing.
- If you are seeking simple comfort or a warming sensation: An unlicensed heat patch or wrap may be sufficient, but do not expect it to deliver a pharmaceutical effect to the underlying tissue.
Ultimately, choosing a licensed medicinal patch means you are treating your pain with a product backed by scientific proof and regulatory oversight.
Summary Table:
| Benefit | Description |
|---|---|
| Verified Efficacy | Contains a proven active ingredient; effectiveness is validated through clinical trials. |
| Assured Safety | Undergoes rigorous safety reviews for side effects and drug interactions. |
| Strict Quality Control | Manufactured to pharmaceutical standards for consistent, accurate dosing. |
| Regulatory Oversight | Approved by health authorities, allowing specific medical claims for pain relief. |
Partner with Enokon for Your Medically Licensed Pain Relief Patches
As a bulk manufacturer of reliable transdermal patches and pain plasters, Enokon provides healthcare and pharmaceutical distributors and brands with a trusted partner for developing OTC or prescription pain relief products. Benefit from our technical expertise for custom R&D, formulation, and development to ensure your patches meet stringent medical licensing standards for efficacy, safety, and quality.
Contact our experts today to discuss your project requirements and leverage our manufacturing excellence.
Visual Guide
Related Products
- Capsaicin Chili Medicated Pain Relief Patches
- Mugwort Wormwood Pain Relief Patch for Neck Pain
- Menthol Gel Pain Relief Patch
- Icy Hot Menthol Medicine Pain Relief Patch
- Lidocaine Hydrogel Pain Relief Patch for Pain Relief
People Also Ask
- Are natural and herbal pain relief patches effective and safe? Discover the Benefits of Targeted Relief
- What is the purpose of capsaicin patches? A Guide to Temporary Pain Relief
- Are pain relief patches safe for sensitive skin? Your Guide to Safe Use & Skin Testing
- Can children use the pain relief patch? A Critical Safety Guide for Parents
- Can the pain relief patch be used with other external analgesic products? A Critical Safety Guide














