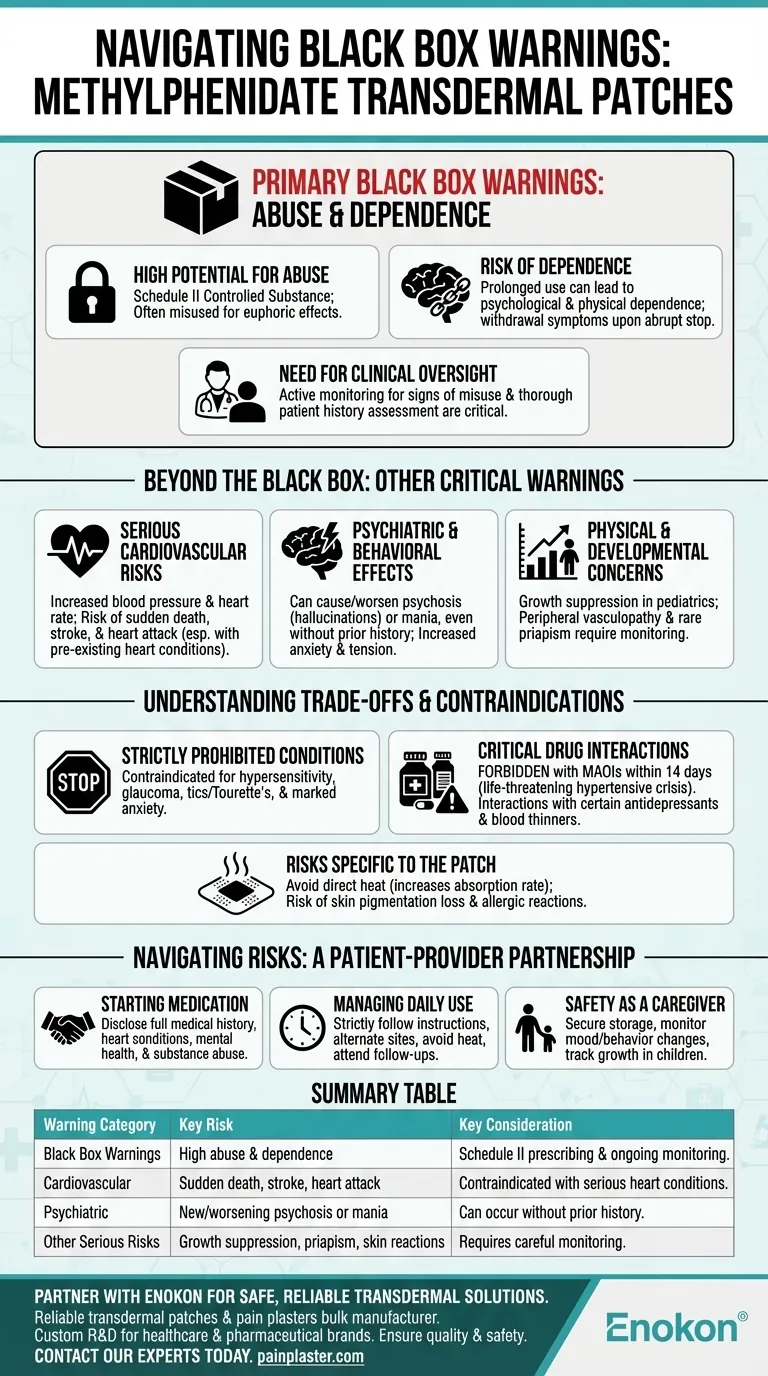
The primary black box warnings for methylphenidate transdermal are its high potential for abuse and dependence. As a central nervous system (CNS) stimulant, this medication carries a significant risk of misuse. Prescribers must assess a patient's risk for abuse before starting therapy and actively monitor for signs of abuse and dependence throughout the course of treatment.
While the official black box warnings focus specifically on abuse and dependence, a complete understanding of safety requires acknowledging the other serious cardiovascular and psychiatric risks associated with this medication. Safe use depends on a thorough evaluation of a patient's full health history.

Deconstructing the Black Box Warnings
The FDA uses black box warnings to highlight the most severe risks associated with a drug. For methylphenidate, these warnings center on its stimulant properties and the potential for misuse.
High Potential for Abuse
Methylphenidate is a Schedule II controlled substance, indicating a high potential for abuse. Abuse occurs when the medication is used in a manner or dosage other than prescribed, often for its euphoric or stimulating effects.
Risk of Dependence
Prolonged use of CNS stimulants can lead to psychological and physical dependence. This means the body may adapt to the drug, leading to withdrawal symptoms if the medication is stopped abruptly and a compulsive need to continue taking it.
The Need for Clinical Oversight
Because of these risks, medical supervision is critical. A healthcare provider will assess a patient's personal and family history of substance abuse before prescribing and will continually monitor for signs of misuse, such as rapid dose escalation or "doctor shopping."
Beyond the Black Box: Other Critical Warnings
Beyond the official black box warnings, several other serious risks and side effects require careful consideration. These warnings underscore the importance of using the medication only under strict medical guidance.
Serious Cardiovascular Risks
Methylphenidate can increase blood pressure and heart rate. There is a documented risk of sudden death, stroke, and heart attack, particularly in individuals with pre-existing structural heart abnormalities or other serious heart problems.
Psychiatric and Behavioral Effects
This medication can cause or worsen certain psychiatric conditions. Patients may experience new or worsening psychosis (e.g., hallucinations, delusional thinking) or manic symptoms, even without a prior history of psychotic illness. Increased anxiety, tension, and agitation are also potential side effects.
Physical and Developmental Concerns
In pediatric patients, growth must be carefully monitored, as long-term stimulant use can be associated with growth suppression. Other notable physical risks include peripheral vasculopathy (including Raynaud's phenomenon) and the rare but serious occurrence of prolonged and painful erections (priapism).
Understanding the Trade-offs and Contraindications
To use this medication safely, it is essential to understand who should not take it and what situations create unacceptable risks.
When Methylphenidate is Strictly Prohibited
This medication is contraindicated (meaning it should not be used) in individuals with certain conditions. These include known hypersensitivity to methylphenidate, glaucoma, motor tics or a diagnosis of Tourette's syndrome, and states of marked anxiety, tension, or agitation.
Critical Drug Interactions
Use of methylphenidate transdermal is strictly forbidden for anyone who has taken a monoamine oxidase inhibitor (MAOI) within the last 14 days. This combination can cause a life-threatening hypertensive crisis. Other medications, including certain antidepressants and blood thinners, may also interact.
Risks Specific to the Transdermal Patch
The patch delivery system introduces unique risks. Users must avoid exposing the application site to direct heat sources (like heating pads or electric blankets), as this can increase the rate of drug absorption. Persistent loss of skin pigmentation and allergic contact sensitization at the application site can also occur.
Making the Right Choice for Your Goal
Navigating the risks of methylphenidate requires a partnership between the patient and the healthcare provider. Open communication and adherence to safety protocols are paramount.
- If your primary focus is starting this medication: Disclose your entire medical history to your doctor, with special attention to any personal or family history of heart conditions, mental health issues, or substance abuse.
- If your primary focus is managing daily use: Strictly follow all application instructions, alternate patch sites, avoid direct heat, and attend all follow-up appointments for monitoring.
- If your primary focus is safety as a caregiver: Securely store the medication out of reach of children, monitor for any changes in mood or behavior, and ensure growth is tracked for pediatric patients.
Understanding the full spectrum of risks, not just the black box warnings, is the first step toward using this medication safely and effectively.
Summary Table:
| Warning Category | Key Risk | Key Consideration |
|---|---|---|
| Black Box Warnings | High potential for abuse and dependence | Requires Schedule II prescribing and ongoing monitoring for misuse. |
| Cardiovascular | Sudden death, stroke, heart attack | Contraindicated in patients with serious pre-existing heart conditions. |
| Psychiatric | New/worsening psychosis or mania | Can occur in patients with or without a prior history. |
| Other Serious Risks | Growth suppression (pediatrics), priapism, severe skin reactions | Requires careful monitoring and patient education. |
Partner with Enokon for Safe, Reliable Transdermal Solutions
Navigating the complex safety profiles of transdermal medications requires a trusted manufacturing partner. As a bulk manufacturer of reliable transdermal patches and pain plasters, Enokon provides healthcare and pharmaceutical distributors and brands with the technical expertise needed for custom R&D and development. We ensure your products meet the highest standards of quality and safety.
Contact our experts today to discuss how we can support your transdermal product development with precision and reliability.
Visual Guide
Related Products
- Icy Hot Menthol Medicine Pain Relief Patch
- Mugwort Wormwood Pain Relief Patch for Neck Pain
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Capsaicin Chili Medicated Pain Relief Patches
- Menthol Gel Pain Relief Patch
People Also Ask
- What additional benefits do self-heating patches provide beyond physical relief? Enhance Well-Being Holistically
- What are some beneficial active ingredients in pain patches? Target Your Pain with the Right Formula
- What is the value of using the dye method to verify emulsion types? Optimize Transdermal Patch R&D and Drug Delivery
- What is the technical purpose of high-simulation placebo patches? Ensure Clinical Integrity for Transdermal Trials
- What are the main advantages of using heat patches for back pain relief? Drug-Free, Targeted Warmth for Lasting Comfort
- What is the disclaimer regarding the use of Moxibustion Patches? Key Safety & Usage Guidelines
- What is the conclusion regarding the choice between testosterone patches and injections? A Personalized Guide
- What risks are associated with concurrent use of smoking or nicotine chewing gum with transdermal nicotine? Avoid a Dangerous Overdose
















