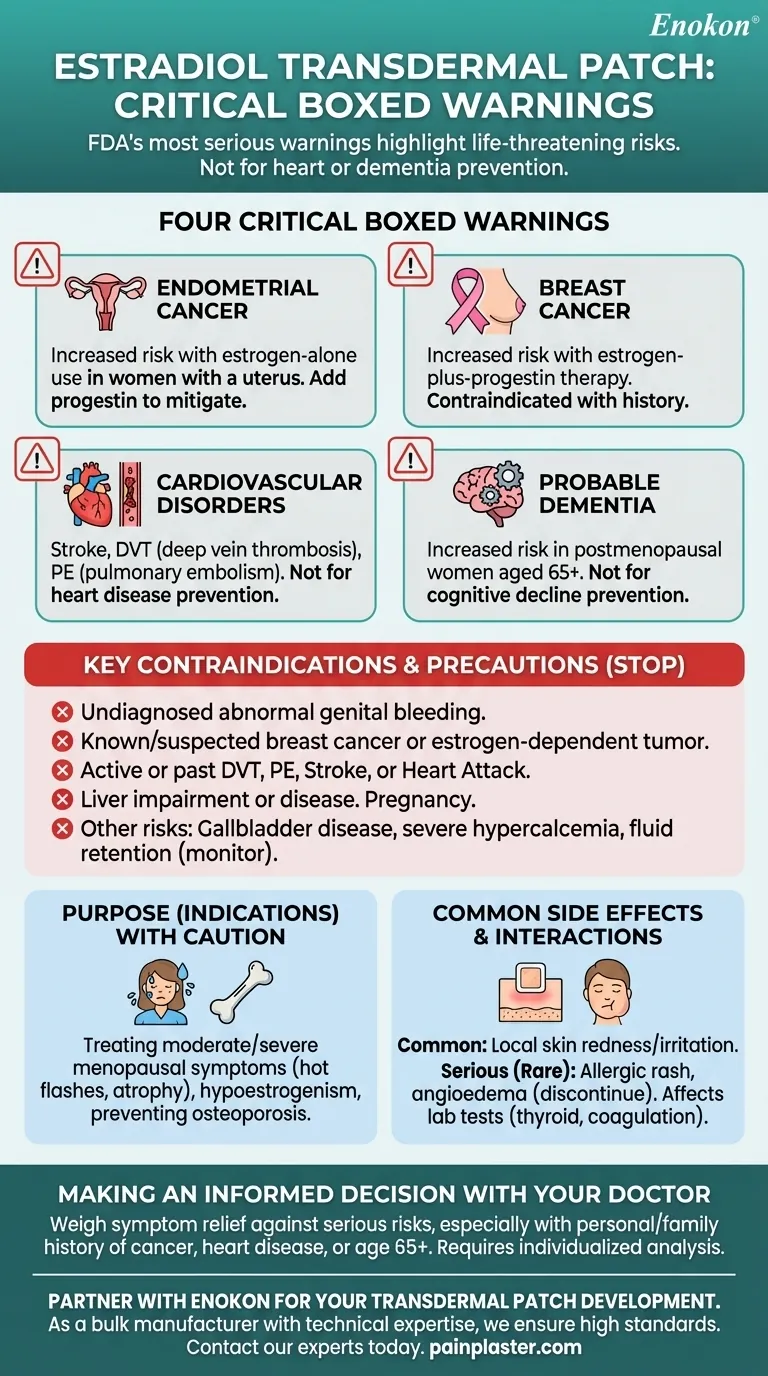
To be clear, the estradiol transdermal patch carries several critical "boxed warnings," which are the most serious type of warning issued by the U.S. Food and Drug Administration (FDA). These warnings highlight potentially life-threatening risks, including an increased risk of endometrial cancer, breast cancer, cardiovascular disorders like stroke and blood clots, and probable dementia.
The central takeaway is that while estradiol can be effective for specific menopausal symptoms, it is not a preventative medicine for heart disease or dementia. Its use introduces significant risks of certain cancers, cardiovascular events, and cognitive decline, which must be carefully weighed against its intended benefits.
Deconstructing the Four Boxed Warnings
Each boxed warning points to a specific, serious risk identified in clinical studies. Understanding the context of each is essential for making an informed health decision.
Increased Risk of Endometrial Cancer
Using estrogen alone in a woman who still has her uterus significantly increases the risk of endometrial cancer (cancer of the uterine lining).
The risk appears to depend on both the duration of treatment and the estrogen dose. To mitigate this risk, it is essential to add a progestin to the hormone therapy regimen.
Increased Risk of Breast Cancer
Studies have shown that the use of estrogen-plus-progestin therapy increases the risk of breast cancer. This is a critical consideration for anyone with a personal or family history of the disease.
The patch is contraindicated for individuals with a known or suspected history of breast cancer or other estrogen-dependent tumors.
Cardiovascular Disorders (Stroke, DVT, PE)
Estrogen therapy is associated with an elevated risk of serious cardiovascular events. It should not be used for the prevention of cardiovascular disease.
These events include:
- Stroke
- Deep Vein Thrombosis (DVT): Blood clots in the deep veins, usually of the legs.
- Pulmonary Embolism (PE): A blood clot that travels to the lungs.
Treatment should be discontinued immediately if any of these events occur or are suspected.
Probable Dementia
For postmenopausal women aged 65 or older, studies show that estrogen-alone or estrogen-plus-progestin therapies increase the risk of developing probable dementia.
This medication is not intended to prevent cognitive decline or memory loss.
Key Contraindications and Precautions
Beyond the boxed warnings, there are specific situations where this medication should not be used (contraindications) and other risks to be aware of.
Who Should Not Use This Medication?
The estradiol patch is strictly contraindicated for individuals with any of the following conditions:
- Undiagnosed abnormal genital bleeding
- Known or suspected breast cancer or other estrogen-dependent neoplasm
- Active DVT, PE, or a history of these conditions
- Active arterial thromboembolic disease (stroke, heart attack) or a history of these
- Known protein C, protein S, or antithrombin deficiency, or other thrombophilic disorders
- Liver impairment or disease
- Pregnancy
Other Important Precautions
Other significant risks include gallbladder disease, severe hypercalcemia (high blood calcium) in patients with cancer, and potential visual abnormalities related to retinal vascular thrombosis.
Conditions aggravated by fluid retention require close monitoring. It is also critical to monitor thyroid function in women on thyroid replacement therapy, as the estrogen may require an increase in their thyroid dose.
Understanding the Trade-offs
The decision to use an estradiol patch involves balancing its intended benefits against its known risks.
The Purpose of Treatment (Indications)
The patch is only approved for specific uses, not general well-being. These indications include:
- Treating moderate to severe vasomotor symptoms of menopause (e.g., hot flashes).
- Treating moderate to severe symptoms of vulvar and vaginal atrophy.
- Treating hypoestrogenism (low estrogen levels).
- Preventing postmenopausal osteoporosis.
Common Side Effects and Interactions
The most common adverse reactions are local skin redness and irritation at the application site. More serious, though rare, reactions can include allergic rash and angioedema (severe swelling), which require immediate and permanent discontinuation of the patch.
This medication can also interfere with the results of certain lab tests, including those for thyroid function, coagulation factors, and glucose tolerance.
Making an Informed Decision with Your Doctor
A thorough discussion of your personal health profile and treatment goals with your healthcare provider is non-negotiable.
- If your primary focus is managing severe menopausal symptoms: You and your doctor must weigh whether the significant relief from symptoms justifies the serious risks outlined in the warnings.
- If you have a personal or family history of cancer, heart disease, or blood clots: This medication may be inappropriate for you, and a comprehensive risk assessment is mandatory before consideration.
- If you are over 65 or concerned about cognitive health: Be aware that this medication is not for preventing dementia and has been shown to increase that specific risk in your age group.
Ultimately, the estradiol transdermal patch is a potent medication that requires a careful, individualized risk-benefit analysis conducted with your healthcare provider.
Summary Table:
| Boxed Warning | Key Risk Details |
|---|---|
| Endometrial Cancer | Risk increases with estrogen-alone use in women with a uterus; mitigated by adding a progestin. |
| Breast Cancer | Risk increases with estrogen-plus-progestin therapy; contraindicated with a history of breast cancer. |
| Cardiovascular Disorders | Increased risk of stroke, DVT, and pulmonary embolism; not for preventing heart disease. |
| Probable Dementia | Risk increases in postmenopausal women aged 65 and older. |
Partner with Enokon for Your Transdermal Patch Development
Navigating complex medication safety profiles requires reliable manufacturing. As a bulk manufacturer of high-quality transdermal patches and pain plasters, Enokon partners with healthcare and pharmaceutical distributors and brands. Benefit from our technical expertise for custom R&D and development, ensuring your products meet the highest standards of safety and efficacy.
Contact our experts today to discuss your project needs.
Visual Guide
Related Products
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Capsaicin Chili Medicated Pain Relief Patches
- Heating Pain Relief Patches for Menstrual Cramps
- Far Infrared Pain Patch Relief Pain Reliever for Back
People Also Ask
- What types of pain can the Deep Heat Pain Relief Back Patch be used for? Targeted Relief for Muscles & Joints
- How do Deep Heat Pain Relief Patches provide pain relief? Discover the Drug-Free Mechanism
- How does capsaicin work in the medicated heat patch? The Science Behind Pain Relief
- What did the UK Million Women Study find regarding transdermal versus oral hormone therapy? A Safer Choice for Gallbladder Health
- How does the Deep Heat Back Patch work? A Drug-Free Solution for Targeted Pain Relief














