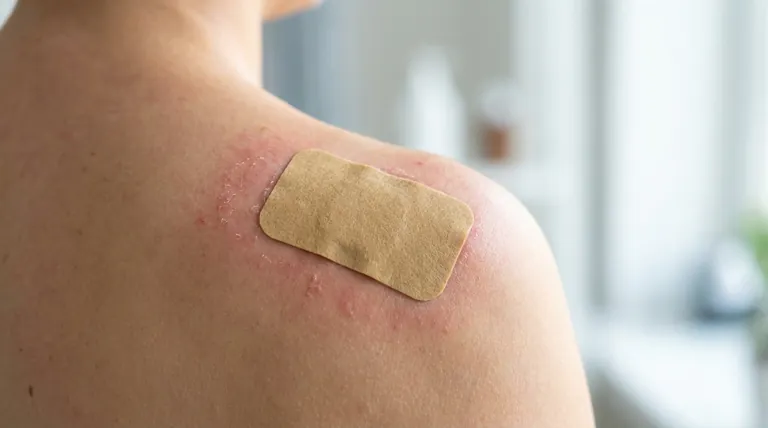
The most common side effects of the transdermal clonidine patch are dermatologic reactions. These range from localized redness, itching, and scaling to more severe allergic responses involving blistering, swelling, and pigmentation changes.
While mild skin irritation is a very common and expected reaction, true allergic contact dermatitis is the most significant dermatologic risk, often forcing patients to discontinue the medication entirely.
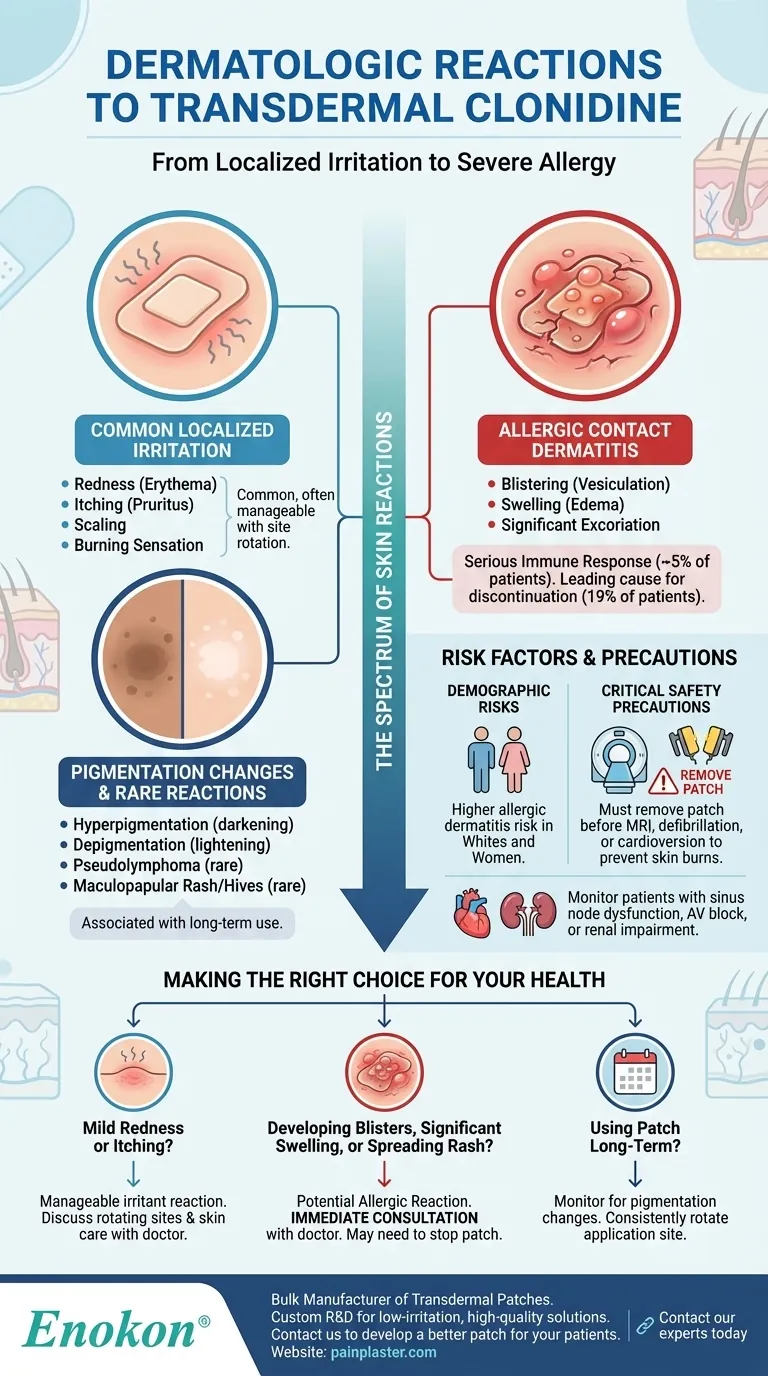
A Spectrum of Skin Reactions: From Irritation to Allergy
Transdermal clonidine delivers medication directly through the skin, which can provoke a variety of reactions at the application site. These can be broadly categorized by their type and severity.
Common Localized Reactions
The most frequent adverse events are localized skin irritations. Studies show erythema (redness) affects a significant portion of patients, with some reports as high as 26%.
Other common reactions include pruritus (itching), scaling, and a burning sensation at the patch site. These symptoms are often a direct result of the patch adhesive or the medication itself irritating the skin.
Allergic Contact Dermatitis
A more serious immune response, allergic contact sensitization, occurs in about 5% of patients. This is a true allergy to a component of the patch system.
Symptoms of an allergic reaction are more pronounced and can include vesiculation (blistering), edema (swelling), and significant excoriation (damage from scratching).
Pigmentation and Other Changes
With long-term use, some patients may experience changes in skin color at the application site. This can manifest as either hyperpigmentation (darkening of the skin) or depigmentation (lightening of the skin).
Rarely, a reaction known as pseudolymphoma has been observed. Other allergic reactions like a maculopapular rash or urticaria (hives) have been reported, though their direct link to the clonidine patch has not been definitively established.
Understanding the Risks and Implications
It is crucial to differentiate between mild irritation and a true allergic reaction, as the implications for continuing treatment are very different.
A Primary Reason for Discontinuation
Dermatologic reactions are not merely a nuisance; they are a leading cause for stopping treatment. Approximately 19% of patients discontinue using the transdermal clonidine system due to contact dermatitis.
Demographic Risk Factors
Evidence suggests that allergic dermatitis from the clonidine patch occurs more commonly in whites and women. Patients in these demographic groups should be particularly mindful of skin changes.
Essential Safety Precautions
Beyond skin reactions, the patch has other important safety considerations. It contains aluminum and must be removed before an MRI, defibrillation, or cardioversion to prevent skin burns.
Patients with sinus node dysfunction, AV block, or renal impairment require careful monitoring when using transdermal clonidine.
Making the Right Choice for Your Health
Understanding these potential reactions empowers you to work with your healthcare provider to manage your treatment effectively.
- If your primary concern is mild redness or itching: This may be a manageable irritant reaction. Discuss rotating patch sites and proper skin care with your physician.
- If you are developing blisters, significant swelling, or a spreading rash: This suggests a potential allergic reaction and requires immediate consultation with your doctor, as you may need to stop using the patch.
- If you are using the patch long-term: Be aware of the possibility of skin pigmentation changes and consistently rotate the application site to minimize this risk.
Proactive monitoring of your skin and open communication with your physician are the keys to using transdermal clonidine safely and effectively.
Summary Table:
| Reaction Type | Common Symptoms | Severity & Implications |
|---|---|---|
| Localized Irritation | Redness (Erythema), Itching (Pruritus), Scaling | Common; often manageable with site rotation |
| Allergic Contact Dermatitis | Blistering, Swelling (Edema), Significant Itching | Serious; leading cause of treatment discontinuation (~19% of patients) |
| Pigmentation Changes | Skin darkening (Hyperpigmentation) or lightening (Depigmentation) | Associated with long-term use |
| Other Reactions | Pseudolymphoma, Maculopapular Rash (rare) | Less common; link to patch may be uncertain |
Need a reliable, dermatologically-tested transdermal patch?
As a bulk manufacturer of transdermal patches and pain plasters, Enokon partners with healthcare and pharma distributors to deliver high-quality, patient-friendly solutions. Our technical expertise ensures custom R&D and development to minimize skin irritation and enhance patient compliance.
Let's develop a better patch for your patients. Contact our experts today to discuss your needs.
Visual Guide
Related Products
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Heating Pain Relief Patches for Menstrual Cramps
- Herbal Eye Protection Patch Eye Patch
- Asthma Cough and Pain Relief Patch for Adults and Kids
People Also Ask
- Are heat patches safe for all body parts? Key Safety Zones and No-Go Areas Explained
- What types of pain can the Deep Heat Pain Relief Back Patch be used for? Targeted Relief for Muscles & Joints
- What did the UK Million Women Study find regarding transdermal versus oral hormone therapy? A Safer Choice for Gallbladder Health
- How does capsaicin work in the medicated heat patch? The Science Behind Pain Relief
- How do Deep Heat Pain Relief Patches provide pain relief? Discover the Drug-Free Mechanism














