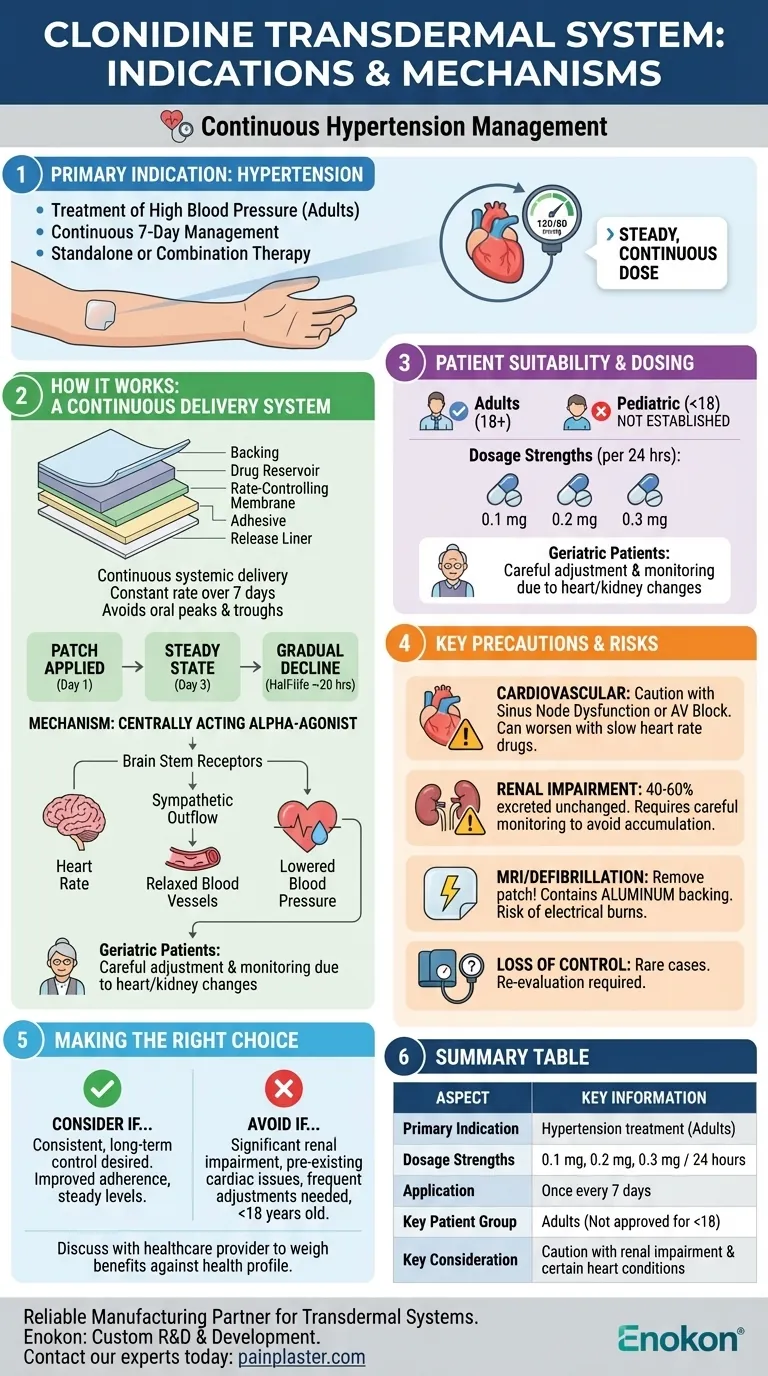
The Clonidine Transdermal System is indicated for one primary purpose: the treatment of hypertension (high blood pressure). It is designed for continuous, long-term management and can be prescribed either as a standalone therapy or in combination with other antihypertensive medications.
This system offers a unique approach to managing hypertension by providing a steady, continuous dose of clonidine over a 7-day period. However, its use requires careful consideration of patient-specific factors, particularly heart and kidney function, to ensure safety and effectiveness.
How the Clonidine Patch Works
The Clonidine Transdermal System is more than just a medication; it's a sophisticated drug delivery device. Understanding its mechanics is key to understanding its application.
A Continuous Delivery System
The system is a multi-layered film patch applied to the skin once every seven days.
This design provides a continuous, systemic delivery of clonidine at a relatively constant rate, avoiding the peaks and troughs associated with oral medications.
The Mechanism of Clonidine
Clonidine is a centrally acting alpha-agonist. It works by stimulating specific receptors in the brain stem.
This action reduces sympathetic outflow from the central nervous system, which in turn decreases heart rate, relaxes blood vessels, and ultimately lowers blood pressure.
Pharmacokinetics: Reaching a Steady State
After applying the patch, it takes approximately three days to reach a stable, or "steady-state," concentration of clonidine in the blood.
Once the patch is removed, the drug's concentration in the plasma declines slowly, with a half-life of about 20 hours. This gradual decline helps prevent rebound hypertension.
Patient Suitability and Dosing
While effective for hypertension, the Clonidine Transdermal System is not suitable for everyone. Proper patient selection is critical.
The Primary Indication: Hypertension
The sole approved indication for this system is the management of high blood pressure in adults.
It is available in several dosage strengths—0.1 mg, 0.2 mg, and 0.3 mg per 24 hours—allowing for tailored treatment based on the patient's needs.
Use in Adult Populations
This medication is intended for use in adults. Its safety and efficacy have not been established in individuals younger than 18 years old.
Special Considerations for Geriatric Patients
While no specific geriatric studies are cited, elderly patients often have age-related changes in heart or kidney function.
Therefore, they may require careful dose adjustments and closer monitoring to avoid potential side effects.
Use in Pediatric Patients
The safety and effectiveness of the Clonidine Transdermal System have not been established in pediatric patients. It is not approved for use in this population.
Understanding Key Precautions and Risks
Objectivity requires acknowledging the potential risks and necessary precautions associated with any treatment.
Cardiovascular Risks
Patients with sinus node dysfunction or atrioventricular (AV) block must use this system with caution. Clonidine can worsen these conditions, especially when used alongside other drugs that slow the heart.
Required Monitoring for Renal Impairment
A significant portion of the absorbed clonidine (40-60%) is excreted unchanged by the kidneys.
Patients with impaired renal function require careful monitoring, as they may not be able to clear the drug effectively, leading to potential accumulation and side effects.
Practical Safety Measures
The backing of the patch contains aluminum. Because of this, the patch must be removed before undergoing an MRI, defibrillation, or cardioversion to prevent the risk of electrical burns.
Loss of Blood Pressure Control
In rare cases, patients may experience a loss of blood pressure control while using the system. This requires a re-evaluation of their treatment plan by a healthcare provider.
Making the Right Choice for Your Treatment Plan
The Clonidine Transdermal System is a valuable tool for specific patient profiles. The decision to use it should be based on a clear understanding of your therapeutic goals.
- If your primary focus is consistent, long-term blood pressure control with weekly dosing: The transdermal system offers a clear advantage over daily oral medications for improving adherence and maintaining steady drug levels.
- If you have significant renal impairment or pre-existing cardiac conduction issues: This therapy requires extreme caution and close medical supervision, and alternative treatments may be more appropriate.
- If you require frequent dose adjustments or are under 18: The slow onset and long half-life of the patch make it less ideal for rapid titration, and it is not approved for pediatric use.
Ultimately, determining if the Clonidine Transdermal System is the right choice requires a thorough discussion with your healthcare provider to weigh its benefits against your individual health profile.
Summary Table:
| Aspect | Key Information |
|---|---|
| Primary Indication | Treatment of hypertension (high blood pressure) in adults. |
| Dosage Strengths | 0.1 mg, 0.2 mg, and 0.3 mg released per 24 hours. |
| Application | Applied to the skin once every 7 days. |
| Key Patient Group | Not approved for use in pediatric patients (<18 years). |
| Key Consideration | Requires caution in patients with renal impairment or certain heart conditions. |
Need a reliable manufacturing partner for transdermal systems like the Clonidine patch?
As Enokon, a bulk manufacturer of reliable transdermal patches and pain plasters, we provide healthcare and pharma distributors and brands with the technical expertise for custom R&D and development. Benefit from our experience to bring your transdermal product to market efficiently and effectively.
Contact our experts today to discuss your project requirements.
Visual Guide
Related Products
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Heating Pain Relief Patches for Menstrual Cramps
- Herbal Eye Protection Patch Eye Patch
- Asthma Cough and Pain Relief Patch for Adults and Kids
People Also Ask
- How does the Deep Heat Back Patch work? A Drug-Free Solution for Targeted Pain Relief
- How does capsaicin work in the medicated heat patch? The Science Behind Pain Relief
- How do Deep Heat Pain Relief Patches provide pain relief? Discover the Drug-Free Mechanism
- Are heat patches safe for all body parts? Key Safety Zones and No-Go Areas Explained
- What did the UK Million Women Study find regarding transdermal versus oral hormone therapy? A Safer Choice for Gallbladder Health















