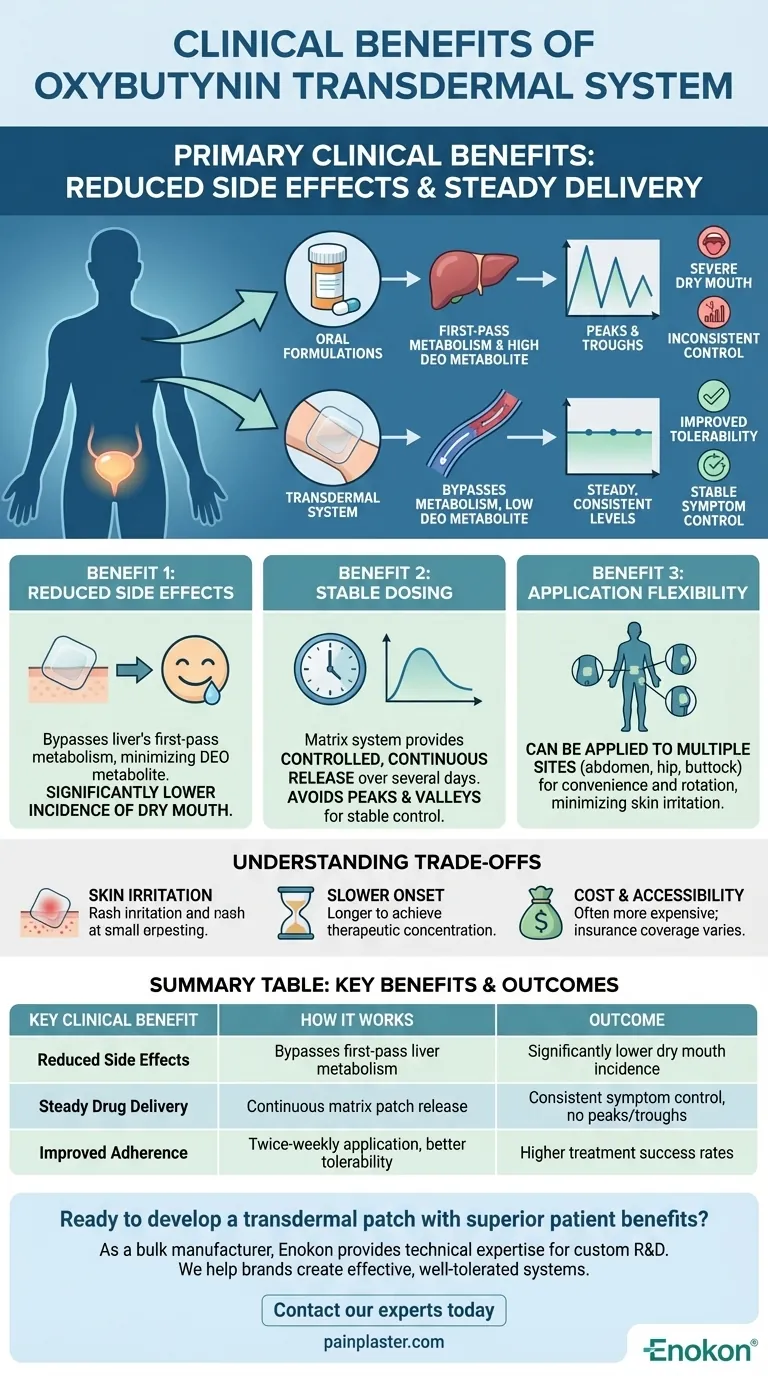
The primary clinical benefits of the oxybutynin transdermal system are a significant reduction in side effects, particularly dry mouth, combined with consistent, steady drug delivery. This is achieved by delivering the medication directly through the skin, which bypasses the digestive system and initial processing by the liver.
The core advantage of the oxybutynin patch is not necessarily increased effectiveness over oral tablets, but a superior side-effect profile. This improved tolerability is the key clinical benefit, often leading to better patient adherence and treatment success.

The Core Challenge: Treating Overactive Bladder
How Oxybutynin Works on Bladder Muscles
Overactive Bladder (OAB) is a condition where the bladder muscles contract uncontrollably. This leads to symptoms like urinary frequency, urgency, and incontinence.
Oxybutynin is an antimuscarinic and antispasmodic agent. It works by relaxing the smooth muscles of the bladder, which decreases these uninhibited contractions.
This action increases the bladder's capacity to hold urine and delays the urgent need to void, directly treating the primary symptoms of OAB.
The Problem with Oral Formulations
When oxybutynin is taken orally, it undergoes extensive "first-pass metabolism" in the liver before it circulates throughout the body.
This process creates a high concentration of a metabolite called N-desethyloxybutynin (DEO). This DEO metabolite is largely responsible for the most common and bothersome side effect of oral oxybutynin: severe dry mouth (xerostomia).
The "peaks and troughs" in drug levels associated with oral dosing can also contribute to inconsistent symptom control and side effects.
How the Transdermal System Changes the Equation
Benefit 1: Bypassing Metabolism to Reduce Side Effects
The transdermal system's greatest benefit is its ability to bypass the liver's first-pass metabolism.
By absorbing directly into the bloodstream through the skin, the patch delivers oxybutynin while minimizing the production of the DEO metabolite.
This directly translates to a much lower incidence of dry mouth, which is a leading reason patients stop taking oral OAB medications.
Benefit 2: Ensuring Stable and Consistent Dosing
The patch is designed as a matrix system that ensures a controlled, continuous release of medication over several days.
This steady diffusion of oxybutynin avoids the sharp peaks and valleys in blood concentration seen with oral pills.
Consistent drug levels provide more stable symptom control throughout the day and night, without the breakthrough symptoms or intense side effects that can occur with fluctuating levels.
Benefit 3: Providing Application Flexibility
The oxybutynin transdermal system can be applied to multiple sites on the body, such as the abdomen, hip, or buttock.
Bioequivalence studies have confirmed that the drug's absorption and reliability are consistent across these different anatomical sites.
This flexibility improves convenience and allows patients to rotate application sites, which can help minimize potential skin irritation.
Understanding the Trade-offs
Skin Irritation
The most common side effect specific to the transdermal patch is localized skin reaction at the application site. This can include redness, itching, or a rash.
This is a known trade-off for many transdermal medications, as the adhesive and drug components are in constant contact with the skin.
Slower Onset of Action
Compared to an immediate-release oral tablet, a transdermal patch takes longer to achieve a therapeutic concentration in the bloodstream. It is not suitable for situations requiring rapid symptom relief.
Cost and Accessibility
Transdermal systems are often more expensive than generic oral formulations of the same medication. Insurance coverage and patient cost can be a significant factor in the selection process.
Making the Right Choice for Your Goal
When deciding on a formulation, the clinical goal and patient profile are paramount.
- If your primary focus is minimizing side effects: The oxybutynin transdermal system is the superior choice, especially for patients who cannot tolerate the dry mouth associated with oral forms.
- If your primary focus is adherence and convenience: The twice-weekly application of a patch can be much easier for patients to manage than a multiple-times-a-day pill regimen.
- If your primary focus is low cost or immediate action: An oral formulation may be the initial choice, with the transdermal system reserved for those who do not tolerate it well.
Choosing the right delivery system is key to balancing therapeutic efficacy with the patient's quality of life.
Summary Table:
| Key Clinical Benefit | How It Works | Outcome |
|---|---|---|
| Reduced Side Effects | Bypasses first-pass liver metabolism, minimizing DEO metabolite. | Significantly lower incidence of dry mouth. |
| Steady Drug Delivery | Continuous release from a matrix patch over several days. | Consistent symptom control, no peaks/troughs. |
| Improved Adherence | Convenient twice-weekly application and better tolerability. | Higher treatment success rates. |
Ready to develop a transdermal patch with superior patient benefits?
As a bulk manufacturer of reliable transdermal patches, Enokon provides the technical expertise for custom R&D and development. We help healthcare and pharma distributors and brands create effective, well-tolerated drug delivery systems.
Contact our experts today to discuss your project and benefit from our experience in transdermal technology.
Visual Guide
Related Products
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Asthma Cough and Pain Relief Patch for Adults and Kids
- Heating Pain Relief Patches for Menstrual Cramps
- Capsaicin Chili Medicated Pain Relief Patches
People Also Ask
- Are heat patches safe for all body parts? Key Safety Zones and No-Go Areas Explained
- What are the key features of the Deep Heat Pain Relief Back Patch? Get Up to 16 Hours of Drug-Free Relief
- Can heat patches be used for fresh injuries? Avoid This Common Mistake for Faster Recovery
- How does capsaicin work in the medicated heat patch? The Science Behind Pain Relief
- How do Deep Heat Pain Relief Patches provide pain relief? Discover the Drug-Free Mechanism
















