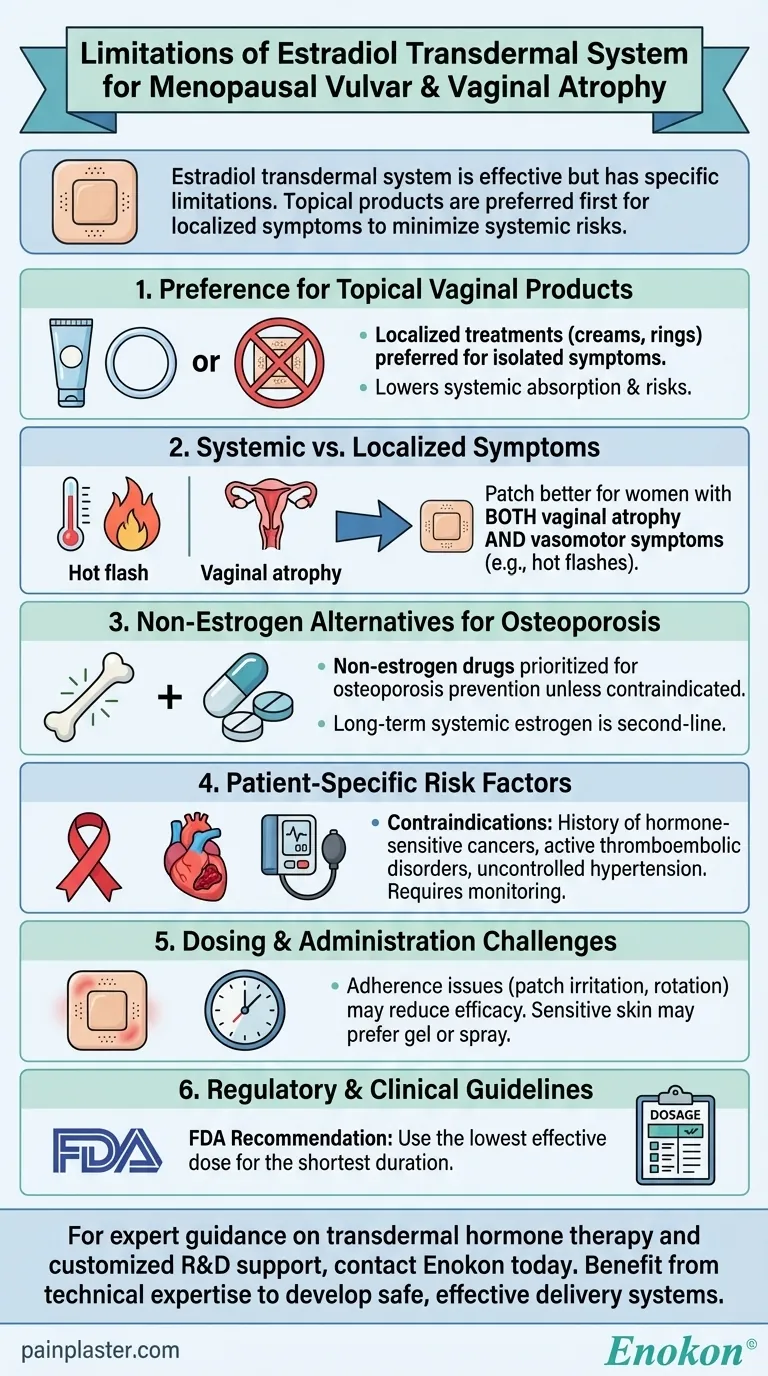
The estradiol transdermal system is effective for treating vulvar and vaginal atrophy due to menopause, but its use has specific limitations. Primarily, topical vaginal products should be considered first for localized symptoms, as systemic estrogen therapy may pose unnecessary risks when only vaginal atrophy is present. The transdermal patch is more suitable for women experiencing additional systemic symptoms like vasomotor issues. Additionally, it should not be the first-line treatment for osteoporosis prevention unless non-estrogen options are unsuitable. Careful patient evaluation is required to balance benefits against potential risks such as cardiovascular events or thromboembolism.

Key Points Explained:
-
Preference for Topical Vaginal Products
- For isolated vulvar/vaginal atrophy, localized treatments (creams, rings) are preferred over systemic Estradiol Transdermal Patch.
- Why? Topical estrogen delivers lower doses directly to affected tissues, minimizing systemic absorption and reducing risks like blood clots or breast cancer associated with oral/transdermal estrogen.
-
Systemic vs. Localized Symptoms
- The patch is better suited for women with both vaginal atrophy and vasomotor symptoms (e.g., hot flashes).
- Example: A patient with severe vaginal dryness and night sweats may benefit more from transdermal estradiol than one with only vaginal discomfort.
-
Non-Estrogen Alternatives for Osteoporosis
- If prescribed solely for osteoporosis prevention, non-estrogen drugs (e.g., bisphosphonates) should be prioritized unless contraindicated.
- Risk Consideration: Long-term systemic estrogen increases stroke and deep vein thrombosis risks, making it a second-line option.
-
Patient-Specific Risk Factors
- Contraindications include:
- History of hormone-sensitive cancers (e.g., breast cancer).
- Active thromboembolic disorders or uncontrolled hypertension.
- Monitoring Required: Regular breast exams and blood pressure checks are essential for users.
- Contraindications include:
-
Dosing and Administration Challenges
- Adherence issues (e.g., patch irritation, rotation sites) may reduce efficacy.
- Practical Tip: Patients with sensitive skin might prefer gel or spray formulations over patches.
-
Regulatory and Clinical Guidelines
- FDA labeling emphasizes using the lowest effective dose for the shortest duration.
- Key Quote: "When prescribing... solely for vaginal atrophy, first consider topical products." (Repeated in references).
By tailoring treatment to symptom type and patient history, clinicians can optimize safety and outcomes. Have you explored how comorbidities like diabetes might influence absorption rates in transdermal delivery? These nuances underscore why individualized care is critical in menopausal hormone therapy.
Summary Table:
| Key Limitation | Explanation |
|---|---|
| Preference for Topical Vaginal Products | Localized treatments (creams, rings) are preferred for isolated symptoms to minimize systemic risks. |
| Systemic vs. Localized Symptoms | Transdermal patches are better suited for women with both vaginal atrophy and vasomotor symptoms. |
| Non-Estrogen Alternatives for Osteoporosis | Non-estrogen drugs should be prioritized for osteoporosis prevention unless contraindicated. |
| Patient-Specific Risk Factors | Contraindications include history of hormone-sensitive cancers and thromboembolic disorders. |
| Dosing and Administration Challenges | Adherence issues like patch irritation may reduce efficacy; alternative formulations may be preferred. |
| Regulatory and Clinical Guidelines | FDA recommends using the lowest effective dose for the shortest duration. |
For expert guidance on transdermal hormone therapy and customized solutions, contact Enokon today. As a trusted bulk manufacturer of transdermal patches and pain plasters, we provide tailored R&D support for healthcare and pharmaceutical brands. Benefit from our technical expertise to develop safe, effective hormone delivery systems.
Visual Guide
Related Products
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Capsaicin Chili Medicated Pain Relief Patches
- Heating Pain Relief Patches for Menstrual Cramps
- Far Infrared Knee Pain Patch Heat Patches for Pain Relief
People Also Ask
- What types of pain can the Deep Heat Pain Relief Back Patch be used for? Targeted Relief for Muscles & Joints
- What are the key features of the Deep Heat Pain Relief Back Patch? Get Up to 16 Hours of Drug-Free Relief
- How do Deep Heat Pain Relief Patches provide pain relief? Discover the Drug-Free Mechanism
- What are the common side effects of using the medicated heat patch? Understanding Risks & Safe Use
- How does the Deep Heat Back Patch work? A Drug-Free Solution for Targeted Pain Relief
















