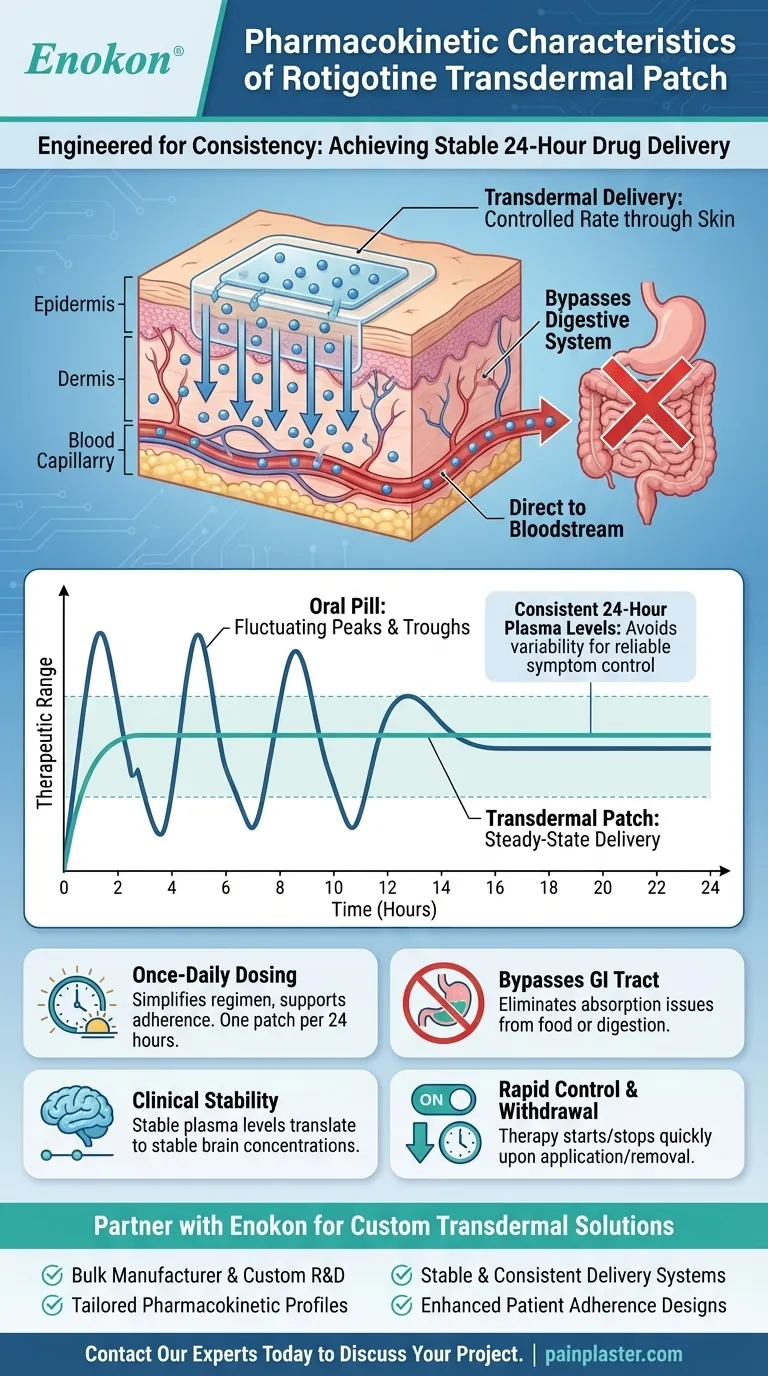
At its core, the rotigotine transdermal patch is engineered for consistency. Its primary pharmacokinetic characteristic is the ability to deliver the drug steadily through the skin, maintaining stable concentrations in the bloodstream over a full 24-hour period with a single daily application.
The crucial advantage of the rotigotine patch is its ability to bypass the digestive system and avoid the fluctuating "peaks and troughs" associated with oral medications. This provides continuous, stable drug delivery for more reliable symptom control.
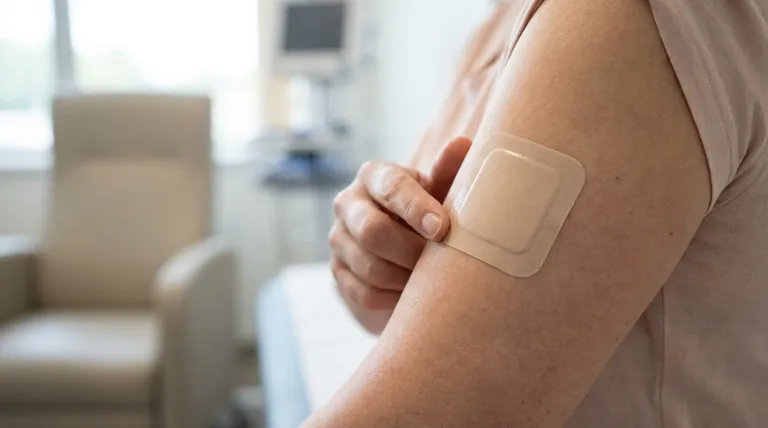
The Principle of Transdermal Delivery
To understand rotigotine's pharmacokinetics, we must first understand how the patch delivery system works. It is fundamentally different from taking a pill.
How the Patch Delivers Medication
A transdermal patch contains a drug reservoir that holds the active rotigotine. This is attached to the skin by an adhesive layer.
The patch is designed to release the drug at a controlled rate through the skin and directly into the network of blood vessels below.
Achieving a Steady State
Unlike an oral pill that causes a spike in drug concentration, the patch provides a slow and continuous infusion. This methodical release allows the body to achieve a stable, steady-state plasma concentration that is maintained for 24 hours.
Bypassing the Digestive System
A significant advantage of this system is that it circumvents gastrointestinal absorption. This eliminates concerns about how food or digestive issues might affect the drug's uptake and effectiveness.
Key Pharmacokinetic Characteristics
The design of the rotigotine patch directly translates into its unique and clinically valuable pharmacokinetic profile.
Consistent 24-Hour Plasma Levels
The hallmark of the rotigotine patch is its ability to maintain stable drug levels in the blood. Animal studies suggest these stable plasma concentrations are also reflected by stable concentrations in the brain.
This continuous delivery helps avoid the variability common with oral dosing, which can lead to periods where the drug level is too high or too low.
Simplified Once-Daily Dosing
Because the patch provides consistent delivery for a full day, it only needs to be applied once every 24 hours. This simplifies the treatment regimen and supports adherence.
Rapid Control and Withdrawal
The transdermal system allows for excellent control. Therapy begins as soon as the patch is applied, and because rotigotine is metabolized rapidly and does not accumulate in the skin, it can be stopped just as quickly by simply removing the patch.
Understanding the Clinical Implications
These pharmacokinetic properties have direct consequences for how the medication is used and the benefits it can provide.
Why Stability Matters
For conditions like Parkinson's disease, fluctuating dopamine agonist levels can lead to unpredictable shifts between "on" periods (good symptom control) and "off" periods (worsening symptoms). Stable drug levels from the patch can help provide smoother, more reliable symptom management.
Use in Special Circumstances
The patch is particularly useful for patients who may have difficulty swallowing pills or are undergoing surgery. It can be applied before, during, or after a surgical procedure to ensure therapy is not interrupted.
Lower Potential for Abuse
While not strictly a pharmacokinetic trait, it is a key characteristic of the delivery system. It is generally more difficult to abuse substances delivered via a transdermal patch compared to other forms of administration.
Applying This to Your Treatment Goal
Choosing a delivery system depends entirely on the clinical objective. The rotigotine patch is specifically designed for situations where consistency is paramount.
- If your primary focus is consistent, all-day symptom control: The patch's ability to maintain stable 24-hour plasma concentrations is its greatest strength.
- If your primary focus is avoiding digestive issues: The transdermal route completely bypasses the GI tract, making it an ideal choice for patients with absorption problems.
- If your primary focus is treatment flexibility: The ability to start and stop therapy simply by applying or removing the patch offers a high degree of control.
Ultimately, the rotigotine patch leverages transdermal technology to provide a reliable and continuous therapeutic effect.
Summary Table:
| Characteristic | Key Benefit |
|---|---|
| Steady-State Delivery | Maintains stable plasma concentration for 24 hours, avoiding peaks and troughs. |
| Bypasses GI Tract | Eliminates variability from food or digestive issues for consistent absorption. |
| Once-Daily Dosing | Simplifies treatment regimen and improves patient adherence. |
| Rapid On/Off Control | Therapy starts upon application and stops upon patch removal. |
Need a reliable transdermal delivery system for your pharmaceutical product?
At Enokon, we are a bulk manufacturer of high-quality transdermal patches and pain plasters. Our technical expertise in custom R&D and development ensures a delivery system tailored to your drug's specific pharmacokinetic profile, just like the rotigotine patch.
We help healthcare and pharma distributors and brands achieve:
- Stable & Consistent Delivery: Engineered for controlled release and reliable 24-hour symptom management.
- Bypass GI Challenges: Ideal for drugs requiring consistent absorption unaffected by digestion.
- Enhanced Patient Adherence: User-friendly, once-daily designs that simplify treatment.
Let's develop your next-generation transdermal solution. Contact our experts today to discuss your project requirements.
Visual Guide
Related Products
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Herbal Eye Protection Patch Eye Patch
- Heating Pain Relief Patches for Menstrual Cramps
- Menthol Gel Pain Relief Patch
- Icy Hot Menthol Medicine Pain Relief Patch
People Also Ask
- How does capsaicin work in the medicated heat patch? The Science Behind Pain Relief
- What are the key features of the Deep Heat Pain Relief Back Patch? Get Up to 16 Hours of Drug-Free Relief
- What are the common side effects of using the medicated heat patch? Understanding Risks & Safe Use
- Are heat patches safe for all body parts? Key Safety Zones and No-Go Areas Explained
- Can heat patches be used for fresh injuries? Avoid This Common Mistake for Faster Recovery













