The clonidine transdermal system is defined by its slow absorption and long duration of action. It has an absolute bioavailability of 60%, but it takes approximately three days of continuous wear to achieve stable, therapeutic plasma concentrations. After the patch is removed, the drug's plasma concentration declines with a half-life of about 20 hours.
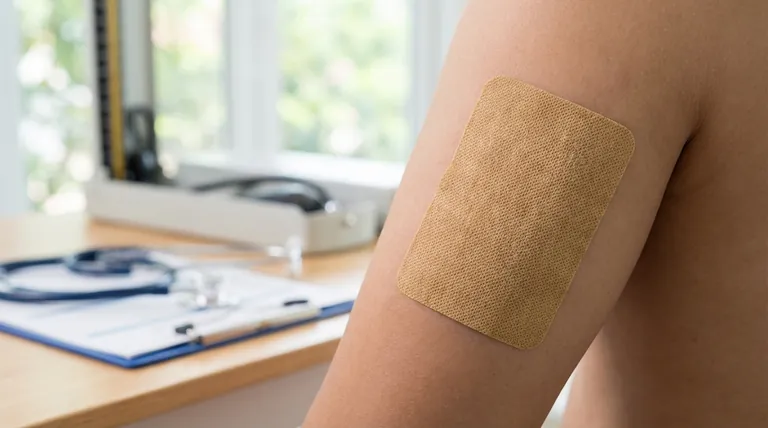
The core principle of the clonidine patch is to mimic a continuous intravenous infusion. This creates exceptionally stable drug levels over a seven-day period, avoiding the blood pressure fluctuations seen with oral dosing, but at the cost of a significantly delayed onset of action.
How the Transdermal System Delivers Clonidine
A Slow and Steady Release
The transdermal system is designed to release clonidine at a constant rate throughout its seven-day application period.
This controlled release transforms the drug's delivery into a pharmacokinetic pattern that closely resembles continuous infusion therapy.
Avoiding Peaks and Troughs
Unlike oral tablets, which cause distinct peaks and troughs in blood concentration after each dose, the patch maintains remarkably consistent plasma levels.
This stability is a key therapeutic advantage, particularly for managing conditions like hypertension where consistent control is paramount.
Key Pharmacokinetic Parameters Explained
Bioavailability and Onset
While the system has a 60% absolute bioavailability, the most critical factor is the time required to reach a therapeutic level.
It takes two to three days for the drug to saturate the skin layers and achieve a steady-state concentration in the bloodstream. This slow onset must be accounted for when initiating therapy.
Dose-Dependent Plasma Levels
The steady-state plasma concentrations are directly proportional to the strength of the patch applied.
Mean concentrations are approximately 0.4 ng/mL for the 0.1 mg/day system, 0.8 ng/mL for the 0.2 mg/day system, and 1.1 ng/mL for the 0.3 mg/day system.
Elimination and Half-Life
Once the patch is removed, clonidine that has been absorbed into the skin continues to be released into the bloodstream.
This creates a "depot effect," resulting in a prolonged elimination half-life of approximately 20 hours. This long tail means the drug's effects will persist for a considerable time after discontinuation.
The Role of Renal Excretion
A significant portion of the absorbed clonidine, about 40-60%, is excreted unchanged in the urine.
Because the kidneys are a primary route of elimination, patients with renal impairment require careful monitoring, as reduced kidney function can lead to drug accumulation.
Understanding the Clinical Trade-offs
The Delayed Onset of Action
The three-day period required to reach steady-state is the system's most significant clinical limitation. The patch is unsuitable for acute treatment and requires a careful transition period when switching from other medications.
Common Dermatological Reactions
The most frequent adverse events are localized to the skin. Reactions include redness, itching, burning, and allergic contact sensitization, which occurs in about 5% of patients.
These skin reactions are a primary reason for treatment discontinuation, with studies showing that up to 19% of patients may stop therapy due to contact dermatitis.
Important Safety Considerations
The patch contains an aluminum backing layer. Therefore, it must be removed before undergoing an MRI, cardioversion, or defibrillation to prevent the risk of electrical arcing and skin burns.
Making the Right Choice for Your Goal
When considering the clonidine transdermal system, its pharmacokinetic profile dictates its ideal use case.
- If your primary focus is stable, long-term blood pressure control: The patch's infusion-like profile offers superior consistency over a 7-day period compared to oral therapy.
- If your primary focus is rapid therapeutic effect: The 3-day delay to reach steady-state makes the transdermal system entirely unsuitable for acute situations.
- If patient adherence or skin sensitivity is a concern: The high rate of dermatological reactions is a significant factor that may lead to treatment discontinuation.
Understanding these pharmacokinetic properties allows for the safe and effective application of the clonidine transdermal system for the appropriate patient.
Summary Table:
| Parameter | Detail |
|---|---|
| Bioavailability | 60% (absolute) |
| Time to Steady State | 2-3 days |
| Elimination Half-Life | ~20 hours |
| Primary Excretion | Renal (40-60% unchanged) |
| Common Adverse Event | Localized skin reactions (up to 19% discontinuation) |
Need a reliable transdermal delivery system for your pharmaceutical product?
At Enokon, we are a bulk manufacturer of high-quality, reliable transdermal patches and pain plasters. Our technical expertise ensures precise, controlled drug release profiles tailored to your therapeutic goals. Whether you are a healthcare distributor or a pharmaceutical brand, we provide custom R&D and development services to bring your product to market efficiently.
Contact our experts today to discuss how we can support your transdermal patch development with consistent, scalable manufacturing.
Visual Guide
Related Products
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Heating Pain Relief Patches for Menstrual Cramps
- Herbal Eye Protection Patch Eye Patch
- Asthma Cough and Pain Relief Patch for Adults and Kids
People Also Ask
- How does the Deep Heat Back Patch work? A Drug-Free Solution for Targeted Pain Relief
- Are heat patches safe for all body parts? Key Safety Zones and No-Go Areas Explained
- What are the key features of the Deep Heat Pain Relief Back Patch? Get Up to 16 Hours of Drug-Free Relief
- Can heat patches be used for fresh injuries? Avoid This Common Mistake for Faster Recovery
- What are the common side effects of using the medicated heat patch? Understanding Risks & Safe Use














