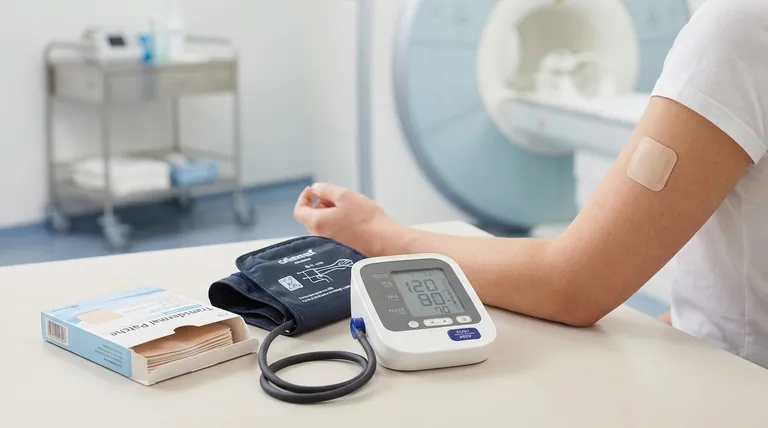
To effectively dose transdermal clonidine, you must understand that it is available in patches designed to deliver 0.1 mg, 0.2 mg, or 0.3 mg per day over a seven-day period. Crucially, there is no established dose-conversion formula when switching from oral clonidine, which necessitates close and careful blood pressure monitoring during the transition.
The core principle of transdermal clonidine is balancing the benefit of steady, continuous drug delivery against the critical need for vigilant monitoring during initiation and awareness of specific safety protocols related to its physical properties.
Core Dosing and Application Principles
Effective use of the clonidine patch begins with understanding its dosage, application, and how to transition patients safely.
Available Dosages
The transdermal system is an extended-release film applied once every seven days. It is available in three strengths, specified by the daily dose delivered: 0.1 mg/24 hr, 0.2 mg/24 hr, and 0.3 mg/24 hr.
Proper Application Technique
To ensure consistent absorption and minimize skin irritation, the patch should be applied to a clean, dry, and hairless area of skin. The recommended application sites are the upper outer arm or the upper torso. It is critical to rotate application sites with each new patch every week.
Switching From Oral Clonidine
A significant practical consideration is the lack of a standardized conversion ratio from oral to transdermal clonidine. This means you cannot simply swap an oral dose for an equivalent patch. When transitioning a patient, close blood pressure monitoring is mandatory to titrate to the correct dose and avoid hypotension or loss of hypertensive control.
Critical Safety and Monitoring Considerations
Beyond initial dosing, several situations require specific precautions to ensure patient safety.
Cardiovascular Precautions
In patients with pre-existing sinus node dysfunction or AV block, transdermal clonidine should be used with caution. The risk is heightened when used concurrently with other sympatholytic drugs.
Managing Special Procedures
The patch contains an aluminum backing layer. Because of this, it must be removed before a patient undergoes an MRI, defibrillation, or cardioversion to prevent the risk of skin burns at the patch site. For patients undergoing surgery, therapy should generally be continued without interruption.
Patients with Renal Impairment
Patients with impaired kidney function require careful monitoring when using transdermal clonidine. The dosage may need to be adjusted based on the clinical response, as clearance of the drug can be affected.
Understanding the Trade-offs and Limitations
While effective, the transdermal patch has practical limitations that influence its use.
Potential for Skin Reactions
As with many transdermal systems, allergic contact dermatitis is a known risk. Patients may experience redness, itching, or rash at the application site, which may necessitate discontinuation.
Rare Loss of Efficacy
In rare instances, patients may experience a loss of blood pressure control even with consistent use of the patch. This requires re-evaluation of their antihypertensive therapy.
The Cost Factor
A significant barrier to the widespread use of transdermal clonidine is its considerably higher cost compared to the oral formulation. This economic factor often limits its application despite its therapeutic advantages, such as improved adherence.
Making the Right Choice for Your Goal
Your approach to dosing should be guided by the specific clinical scenario.
- If your primary focus is initiating therapy or switching from oral: Prioritize gradual titration and frequent blood pressure monitoring due to the lack of a direct dose conversion.
- If your primary focus is managing long-term therapy: Emphasize consistent weekly application and diligent rotation of the patch site to maintain efficacy and prevent skin irritation.
- If your primary focus is patient safety during procedures: Always remember to remove the patch before an MRI or cardioversion to prevent burns from its aluminum content.
Ultimately, mastering transdermal clonidine involves leveraging its benefit of steady delivery while proactively managing its unique application and safety requirements.
Summary Table:
| Consideration | Key Point |
|---|---|
| Available Strengths | 0.1 mg, 0.2 mg, or 0.3 mg per day (7-day patch) |
| Switching from Oral | No standard conversion; requires close BP monitoring |
| Critical Safety | Remove patch before MRI/cardioversion; risk of skin irritation |
| Key Limitation | Higher cost compared to oral formulation |
Need a reliable partner for your transdermal patch development?
As Enokon, a bulk manufacturer of reliable transdermal patches and pain plasters, we provide healthcare and pharma distributors and brands with expert technical support for custom R&D and development. Benefit from our expertise to create effective and safe transdermal solutions.
Contact our team today to discuss your project requirements.
Visual Guide
Related Products
- Capsaicin Chili Medicated Pain Relief Patches
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Heating Pain Relief Patches for Menstrual Cramps
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Herbal Eye Protection Patch Eye Patch
People Also Ask
- What precautions should be taken with buprenorphine patches? Ensure Safe Use and Avoid Overdose Risks
- Are pain relief patches safe for sensitive skin? Your Guide to Safe Use & Skin Testing
- What side effects might occur from using capsaicin patches? Understand the difference between normal sensation and danger signs.
- Are natural and herbal pain relief patches effective and safe? Discover the Benefits of Targeted Relief
- How do pain relief patches work? A Guide to Targeted, Long-Lasting Pain Relief














