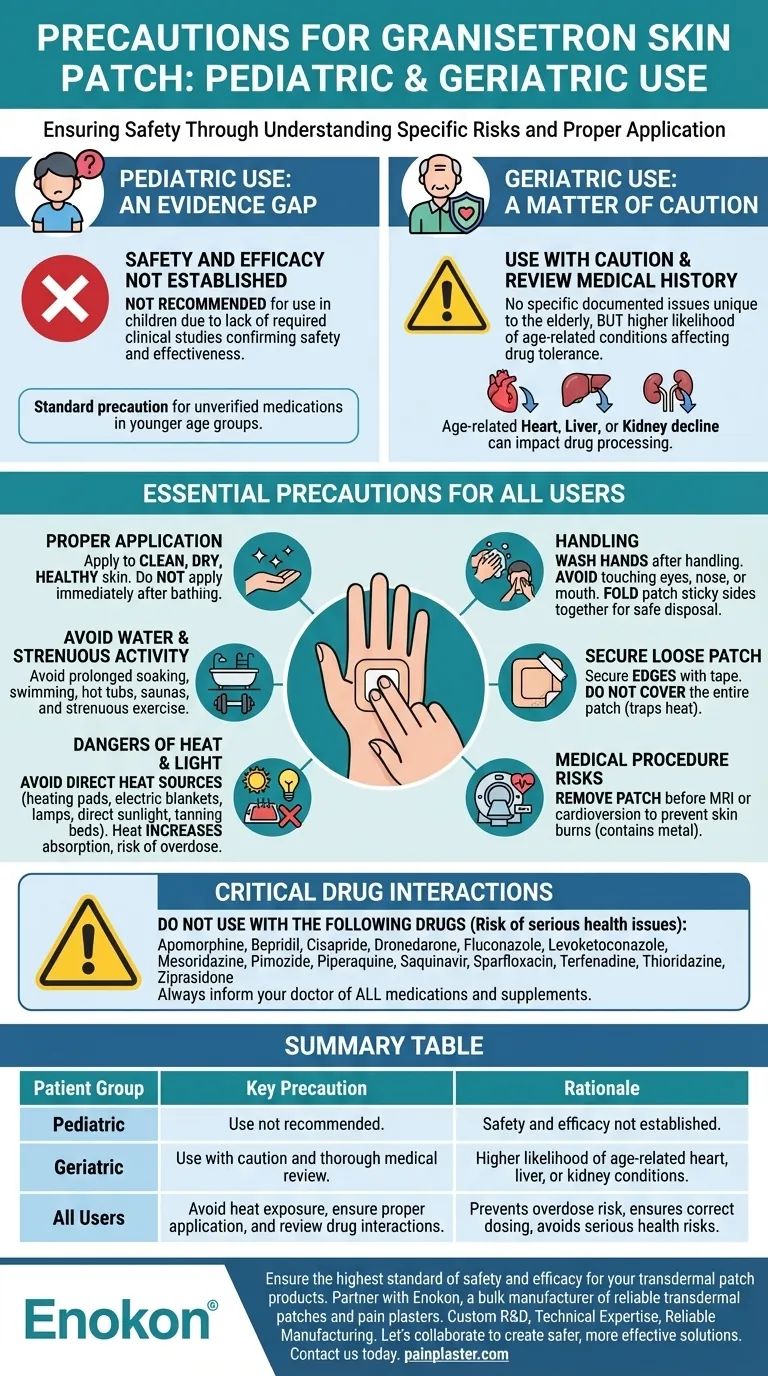
For pediatric patients, the safety and effectiveness of the granisetron skin patch have not been established through appropriate studies. For geriatric patients, while no specific problems have been documented, caution is advised as elderly individuals are more likely to have age-related heart, liver, or kidney conditions that can affect how the medication is tolerated.
The central challenge with the granisetron patch is a lack of safety data in children and the need for careful monitoring in the elderly due to potential underlying health issues. For all patients, safety hinges on correct application and avoiding specific environmental factors like heat that can dangerously alter medication absorption.

Pediatric Use: An Evidence Gap
Safety and Efficacy Not Established
Clinical studies required to confirm that the granisetron skin patch is both safe and effective for use in children have not been performed.
Due to this lack of data, its use in the pediatric population is not recommended. This is a standard precaution for medications that have not been specifically tested in younger age groups.
Geriatric Use: A Matter of Caution
No Specific Documented Issues
To date, studies have not identified any problems that are unique to the geriatric population when using the granisetron patch. This suggests it can be as effective in older adults as it is in younger adults.
The Role of Age-Related Conditions
The primary concern for elderly patients is the higher likelihood of pre-existing medical conditions. Age-related decline in heart, liver, or kidney function can impact how the body processes medications.
Therefore, while the patch itself isn't a specific problem, a thorough medical history is crucial to ensure it is used safely in the context of a patient's overall health.
Essential Precautions for All Users
Proper Application and Handling
Always apply the patch to clean, dry, and healthy skin. To ensure proper adhesion and safety, do not apply it immediately after bathing when the skin may be moist.
After handling the patch, wash your hands thoroughly and avoid touching your eyes, nose, or mouth. When a patch is removed, it should be folded with the sticky sides together and disposed of safely away from children or pets.
Avoiding Water and Strenuous Activity
While wearing the patch, you should avoid activities that could compromise its adhesion or alter drug delivery. This includes prolonged soaking in a bath, swimming, using whirlpools or saunas, and strenuous exercise.
Securing a Loose Patch
If a patch begins to loosen, you can secure the edges with medical adhesive tape or a surgical bandage. However, it is critical not to cover the entire patch, as this can trap heat and affect medication absorption.
Understanding Critical Risks and Interactions
The Dangers of Heat and Light Exposure
You must not expose the patch or the surrounding skin to direct heat sources. This includes heating pads, electric blankets, heat lamps, and direct sunlight, as heat can significantly increase the amount of medication absorbed into the bloodstream, leading to potential overdose.
The patch application site should be protected from sunlight and artificial light sources (like tanning beds) while wearing it and for a period after removal.
Medical Procedure Contraindications
The patch contains metal components and must be removed before undergoing certain medical procedures. It poses a risk of skin burns if worn during a Magnetic Resonance Imaging (MRI) scan or cardioversion.
Critical Drug Interactions
Combining the granisetron patch with certain other medications can cause serious health risks. It should not be used with the following drugs:
- Apomorphine
- Bepridil
- Cisapride
- Dronedarone
- Fluconazole
- Levoketoconazole
- Mesoridazine
- Pimozide
- Piperaquine
- Saquinavir
- Sparfloxacin
- Terfenadine
- Thioridazine
- Ziprasidone
Always inform your doctor and pharmacist of all prescription drugs, over-the-counter products, and supplements you are using.
Making the Right Choice for Patient Safety
- If your primary focus is safety for a child: The granisetron patch is not recommended, as its safety and efficacy have not been studied in this population.
- If your primary focus is safety for an elderly patient: Proceed with caution and ensure their doctor has a complete picture of their health, particularly any heart, kidney, or liver conditions.
- If your primary focus is general safe use for any patient: Meticulously follow all application, handling, and environmental precautions, especially avoiding heat exposure and discussing all other medications with a healthcare provider.
Ultimately, ensuring patient safety requires a clear understanding of both the user's health profile and the medication's specific operational risks.
Summary Table:
| Patient Group | Key Precaution | Rationale |
|---|---|---|
| Pediatric | Use not recommended. | Safety and efficacy not established in clinical studies. |
| Geriatric | Use with caution and thorough medical review. | Higher likelihood of age-related heart, liver, or kidney conditions affecting drug tolerance. |
| All Users | Avoid heat exposure, ensure proper application, and review drug interactions. | Prevents overdose risk, ensures correct dosing, and avoids serious health risks. |
Ensure the highest standard of safety and efficacy for your transdermal patch products.
At Enokon, we are a bulk manufacturer of reliable transdermal patches and pain plasters. We understand that developing safe medications for sensitive populations like pediatric and geriatric patients requires meticulous attention to detail and robust technical expertise.
Partner with us to benefit from:
- Custom R&D and Development: Tailor formulations to meet specific safety and efficacy profiles for your target patient groups.
- Technical Expertise: Leverage our deep knowledge to navigate complex regulatory requirements and optimize product design.
- Reliable, High-Quality Manufacturing: Ensure consistent delivery of patches that meet the highest standards for healthcare/pharma distributors and brands.
Let's collaborate to create safer, more effective transdermal solutions. Contact our experts today to discuss your project needs.
Visual Guide
Related Products
- Natural Herbal Wormwood Patch Pain Plaster
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Capsaicin Chili Medicated Pain Relief Patches
- Mugwort Wormwood Pain Relief Patch for Neck Pain
- Lidocaine Hydrogel Pain Relief Patch for Pain Relief
People Also Ask
- What are pain relief patches? Discover Targeted, Drug-Free Pain Management Solutions
- Are pain relief patches safe for sensitive skin? Your Guide to Safe Use & Skin Testing
- What medical conditions should be reported before using buprenorphine patches? Essential Safety Guide
- How do pain relief patches provide targeted relief? Discover the Science Behind Effective Pain Management
- Can pregnant women use pain relief patches? Your Essential Guide to Safe Pain Management














