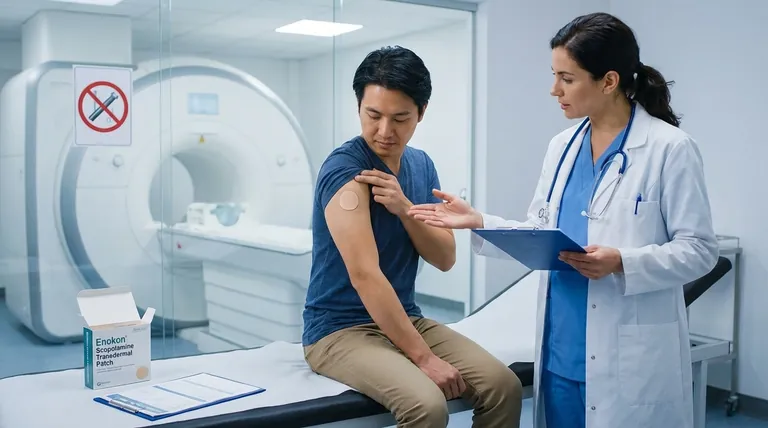
Scopolamine transdermal patches, commonly used for motion sickness and nausea, require specific warnings regarding medical tests due to potential interference with results and safety risks. Key concerns include MRI-related skin burns, altered test outcomes, and serious side effects that may mimic or complicate diagnostic evaluations. Patients must inform healthcare providers about patch use before any medical procedures or tests to ensure accurate interpretations and prevent adverse events.
Key Points Explained:
-
MRI Safety Hazard
- The Scopolamine Transdermal Patch contains metal components that can overheat during MRI scans, causing skin burns.
- Action Required: Remove the patch before undergoing MRI procedures and reapply afterward if needed.
-
Interference with Medical Test Results
- Scopolamine may skew results of:
- Pupillary response tests (due to anticholinergic effects causing dilated pupils).
- Gastric motility studies (as it slows digestion).
- Cognitive/neurological assessments (risk of confusion or hallucinations).
- Action Required: Disclose patch use to all healthcare providers and lab personnel prior to testing.
- Scopolamine may skew results of:
-
Drug Interaction Risks During Testing
- Concurrent use with phosphodiesterase inhibitors (e.g., sildenafil) may cause severe hypotension during cardiovascular tests.
- Anesthesia interactions could alter vital sign monitoring.
- Action Required: Review all medications with the care team before invasive procedures.
-
Side Effects Mimicking Clinical Conditions
- Severe reactions like hallucinations, extreme dizziness, or urinary retention may be misinterpreted as:
- Stroke symptoms
- Psychiatric episodes
- Prostate/renal dysfunction
- Action Required: Differentiate drug effects from emergent conditions by verifying patch use timeline.
- Severe reactions like hallucinations, extreme dizziness, or urinary retention may be misinterpreted as:
-
Special Populations Requiring Vigilance
- Elderly patients are prone to heightened side effects that could complicate diagnostic accuracy.
- Children using the patch (off-label) need growth monitoring due to potential systemic effects.
-
Pre-Test Preparation Protocol
- For elective tests, consider temporary discontinuation under medical supervision.
- For emergency testing, document patch placement time/location to assess burn risks with electrocautery or defibrillation.
Reflective Consideration: How might invisible medication patches impact routine care workflows? These silent drug delivery systems demand proactive communication between patients and providers to avoid diagnostic pitfalls.
By addressing these warnings systematically, healthcare teams can mitigate risks while leveraging scopolamine’s therapeutic benefits—a reminder of how small devices carry significant clinical implications.
Summary Table:
| Warning Category | Key Risks | Action Required |
|---|---|---|
| MRI Safety Hazard | Metal components may cause skin burns during MRI scans. | Remove patch before MRI; reapply after if needed. |
| Test Interference | Skews pupillary, gastric, and cognitive tests due to anticholinergic effects. | Disclose patch use to providers before testing. |
| Drug Interactions | Severe hypotension with phosphodiesterase inhibitors; anesthesia interference. | Review all medications with care team pre-procedure. |
| Side Effect Mimicry | Hallucinations/dizziness may resemble stroke or psychiatric episodes. | Verify patch use timeline to differentiate from emergent conditions. |
| Special Populations | Elderly/children at higher risk for complications. | Monitor closely; consider discontinuation for elective tests under supervision. |
Ensure Patient Safety with Expert-Grade Transdermal Solutions
Navigating scopolamine patch risks requires precision and expertise. Partner with Enokon, a trusted bulk manufacturer of transdermal patches and pain plasters for healthcare distributors and brands. Our technical team supports:
- Custom R&D to optimize patch formulations for reduced interference risks.
- Regulatory-compliant production ensuring safety in MRI/test environments.
- White-label solutions tailored to your clinical needs.
Contact us today to discuss safer transdermal delivery systems for your patients.
Visual Guide

Related Products
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Heating Pain Relief Patches for Menstrual Cramps
- Herbal Eye Protection Patch Eye Patch
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Capsaicin Chili Medicated Pain Relief Patches
People Also Ask
- What are the key features of the Deep Heat Pain Relief Back Patch? Get Up to 16 Hours of Drug-Free Relief
- How quickly does the Deep Heat Pain Relief Back Patch activate and how long does it provide warmth? Get 16-Hour Relief
- What did the UK Million Women Study find regarding transdermal versus oral hormone therapy? A Safer Choice for Gallbladder Health
- How does capsaicin work in the medicated heat patch? The Science Behind Pain Relief
- How does the Deep Heat Back Patch work? A Drug-Free Solution for Targeted Pain Relief













