The primary concerns that prompted revised labeling for the contraceptive patch were its higher estrogen exposure compared to typical birth control pills and subsequent reports linking it to an increased risk of serious blood clots, a condition known as venous thromboembolism (VTE). The new labeling was added to ensure users and prescribers were fully aware of this specific potential risk.
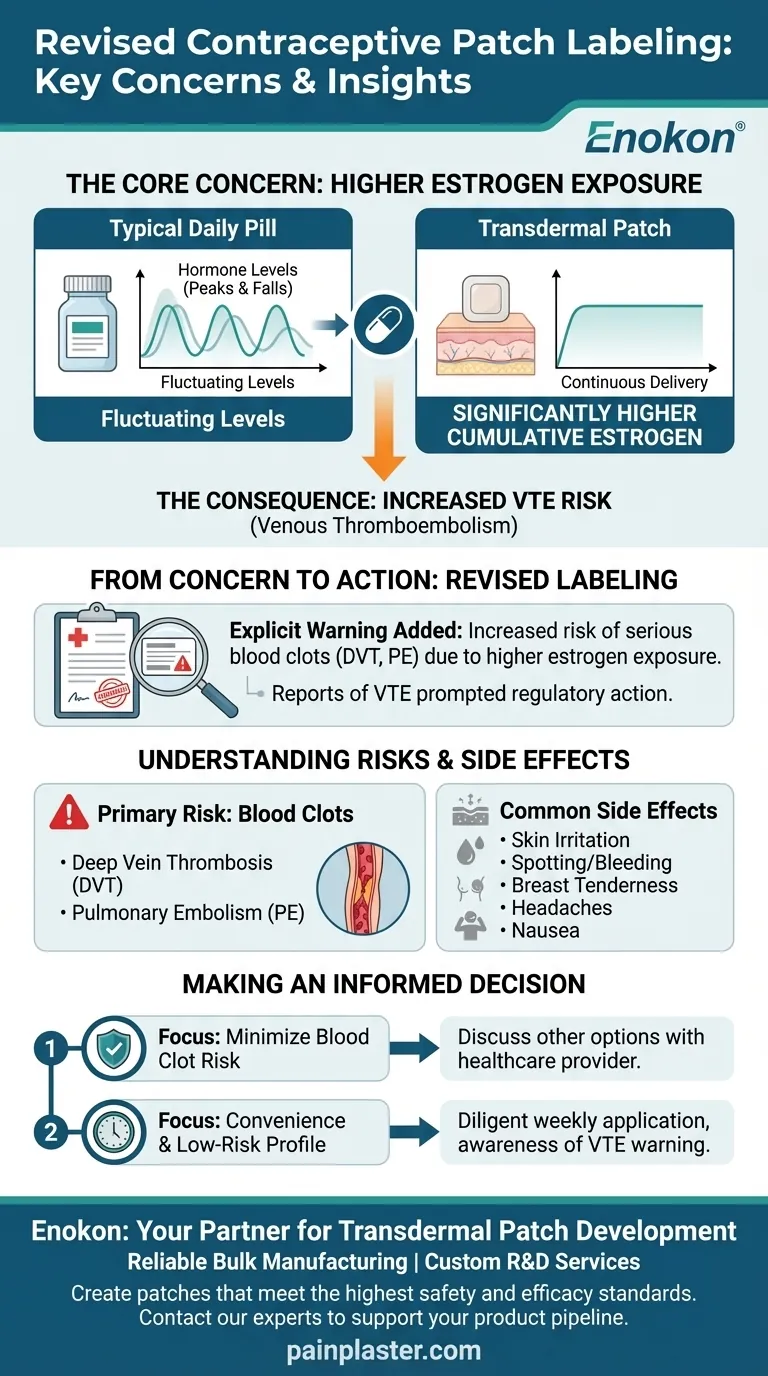
The central issue is that the transdermal patch delivers a continuous and higher overall dose of estrogen into the bloodstream than many common oral contraceptives. This elevated estrogen level is the mechanism linked to a potentially greater risk of developing dangerous blood clots.

The Core Safety Concern: Estrogen Exposure
Why Estrogen Levels Matter
The hormone estrogen, a key component in most combination contraceptives, is known to affect blood clotting factors.
Higher, sustained levels of estrogen can increase the risk of forming blood clots in the veins, particularly in the legs or lungs. This is the underlying principle behind the safety evaluation.
The Patch's Unique Delivery System
Unlike a daily pill which causes hormone levels to peak and fall, the patch delivers a steady, continuous dose of hormones through the skin.
This method results in a user's body being exposed to significantly higher cumulative levels of estrogen over time compared to someone taking a typical combination birth control pill.
From Concern to Action: The Labeling Change
The Link to Venous Thromboembolism (VTE)
Regulatory bodies took action after receiving and analyzing reports of VTE—a serious condition that includes deep vein thrombosis (DVT) and pulmonary embolism (PE)—among patch users.
These reports raised a clear signal that the risk profile for the patch might be different from that of other hormonal methods.
The Specific Warning
The revised product labeling now includes an explicit warning.
It states that women who use the contraceptive patch may be at an increased risk for developing venous thromboembolism due to the higher estrogen exposure when compared to women using certain oral contraceptives.
Understanding the Trade-offs and Side Effects
The Primary Risk: Blood Clots
The most serious potential risk associated with the patch is the increased possibility of VTE. This concern is the entire basis for the labeling change and should be the primary consideration in any risk-benefit discussion with a healthcare provider.
Common Side Effects
Beyond the risk of VTE, the patch shares many common side effects with other hormonal contraceptives.
These can include skin irritation at the application site, spotting or bleeding between periods, breast tenderness, headaches, and nausea.
Considerations for Effectiveness
The patch is a highly effective contraceptive method, but its efficacy depends on correct and consistent use.
Forgetting to change the patch on schedule each week significantly reduces its effectiveness in preventing pregnancy.
Making an Informed Decision
Understanding the specific risk profile of any medication is key. The labeling change for the contraceptive patch was designed to empower you and your doctor to make the best choice based on your individual health.
- If your primary focus is minimizing risk factors for blood clots: You must discuss the patch's higher estrogen exposure with your doctor, as other contraceptive options may be more appropriate.
- If your primary focus is convenience and you have a low-risk health profile: The patch can be an effective option, but you must be diligent about weekly changes and aware of both the common side effects and the specific VTE warning.
Ultimately, this information allows for a more transparent conversation about balancing contraceptive effectiveness with personal safety.
Summary Table:
| Concern | Reason for Labeling Change |
|---|---|
| Higher Estrogen Exposure | The patch delivers a continuous, higher cumulative dose than many oral contraceptives. |
| Increased VTE Risk | Higher estrogen levels are linked to a greater risk of venous thromboembolism (blood clots). |
Need a reliable partner for transdermal patch development?
At Enokon, we are a bulk manufacturer of reliable transdermal patches and pain plasters for healthcare and pharma distributors and brands. Our technical expertise ensures your products are developed with precision, safety, and efficacy in mind.
Benefit from our custom R&D and development services to create patches that meet the highest standards.
Contact our experts today to discuss your specific requirements and how we can support your product pipeline.
Visual Guide
Related Products
- Prostate Pain Kidney Health Care Patch for Men
- Capsaicin Chili Medicated Pain Relief Patches
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Lidocaine Hydrogel Pain Relief Patch for Pain Relief
- Icy Hot Menthol Medicine Pain Relief Patch
People Also Ask
- What should patients tell their doctor before using testosterone patches? A Guide to Safe Treatment
- What should be done if a dose of testosterone patches is missed? Regain Stability and Safety
- What lifestyle factors should be considered when choosing between testosterone patches and injections? Find Your Best Fit
- What should be done in case of a testosterone patch overdose? A Step-by-Step Emergency Guide
- What is the purpose of testosterone patches? A Steady Solution for Low Testosterone















