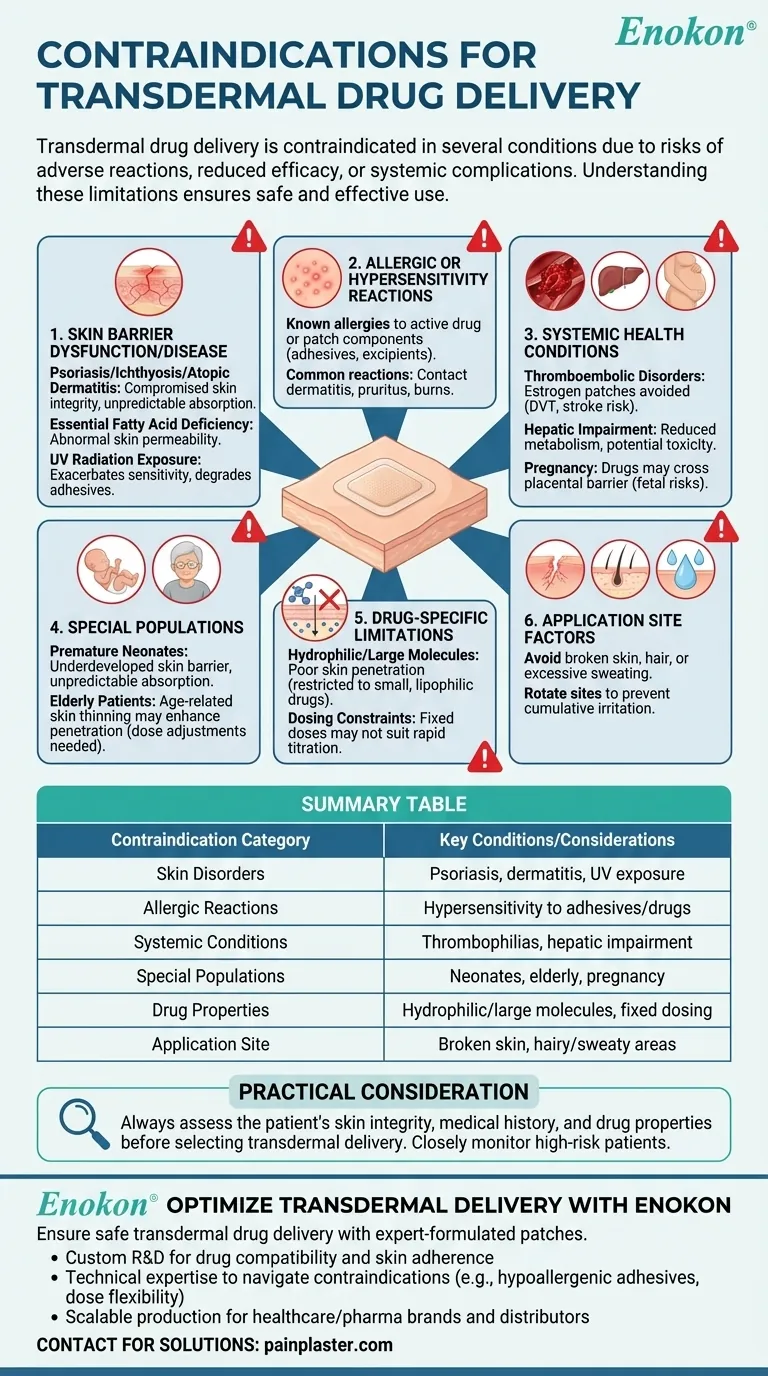
Transdermal drug delivery via transdermal drug patches is contraindicated in several conditions due to risks of adverse reactions, reduced efficacy, or systemic complications. Key contraindications include skin disorders (e.g., psoriasis, dermatitis), systemic conditions (e.g., thrombophilias, hepatic impairment), and patient-specific factors (e.g., pregnancy, neonatal status). Understanding these limitations ensures safe and effective use of this delivery method.
Key Points Explained:
-
Skin Barrier Dysfunction or Disease
- Psoriasis/Ichthyosis/Atopic Dermatitis: These conditions compromise skin integrity, increasing absorption unpredictably and raising risks of systemic toxicity or localized irritation.
- Essential Fatty Acid Deficiency: Leads to abnormal skin permeability, altering drug absorption rates.
- UV Radiation Exposure: Can exacerbate skin sensitivity or degrade patch adhesives/drug stability.
-
Allergic or Hypersensitivity Reactions
- Contraindicated in patients with known allergies to the active drug or patch components (e.g., adhesives, excipients). Common reactions include contact dermatitis, pruritus, or burns.
-
Systemic Health Conditions
- Thromboembolic Disorders: Estrogen-containing patches (e.g., hormonal therapy) are avoided in patients with a history of DVT, stroke, or thrombophilias due to increased clotting risks.
- Hepatic Impairment: Impaired liver function may reduce drug metabolism, leading to accumulation and toxicity.
- Pregnancy: Many drugs (e.g., hormones, opioids) can cross the placental barrier, posing fetal risks.
-
Special Populations
- Premature Neonates: Their underdeveloped skin barrier increases systemic absorption unpredictably.
- Elderly Patients: Age-related skin thinning may enhance drug penetration, requiring dose adjustments.
-
Drug-Specific Limitations
- Hydrophilic/Large Molecules: Poor skin penetration restricts use to small, lipophilic drugs (e.g., fentanyl, nicotine).
- Dosing Constraints: Fixed patch doses may not suit patients needing rapid titration.
-
Application Site Factors
- Avoid areas with broken skin, hair, or excessive sweating to ensure adhesion and consistent absorption. Rotate sites to prevent cumulative irritation.
Practical Consideration: Always assess the patient’s skin integrity, medical history, and drug properties before selecting transdermal delivery. For example, a patient with psoriasis may tolerate oral alternatives better, while those with hepatic impairment might require closer monitoring.
By addressing these contraindications, clinicians can mitigate risks while leveraging the benefits of sustained, non-invasive drug delivery.
Summary Table:
| Contraindication Category | Key Conditions/Considerations |
|---|---|
| Skin Disorders | Psoriasis, dermatitis, UV exposure |
| Allergic Reactions | Hypersensitivity to adhesives/drugs |
| Systemic Conditions | Thrombophilias, hepatic impairment |
| Special Populations | Neonates, elderly, pregnancy |
| Drug Properties | Hydrophilic/large molecules, fixed dosing |
| Application Site | Broken skin, hairy/sweaty areas |
Ensure safe transdermal drug delivery with expert-formulated patches — contact Enokon for tailored solutions. As a bulk manufacturer of reliable transdermal patches and pain plasters, we provide:
- Custom R&D for drug compatibility and skin adherence
- Technical expertise to navigate contraindications (e.g., hypoallergenic adhesives, dose flexibility)
- Scalable production for healthcare/pharma brands and distributors
Let’s optimize your transdermal delivery system together.
Visual Guide
Related Products
- Natural Herbal Wormwood Patch Pain Plaster
- Icy Hot Menthol Medicine Pain Relief Patch
- Capsaicin Chili Medicated Pain Relief Patches
- Mugwort Wormwood Pain Relief Patch for Neck Pain
- Heating Pain Relief Patches for Menstrual Cramps
People Also Ask
- What was the reported pain relief after the initial month of plaster use? Consistent & Effective Pain Management
- How do pain relief patches provide targeted relief? Discover the Science Behind Effective Pain Management
- What medical conditions should be reported before using buprenorphine patches? Essential Safety Guide
- Can pregnant women use pain relief patches? Your Essential Guide to Safe Pain Management
- How do pain relief patches work? A Guide to Targeted, Long-Lasting Pain Relief















