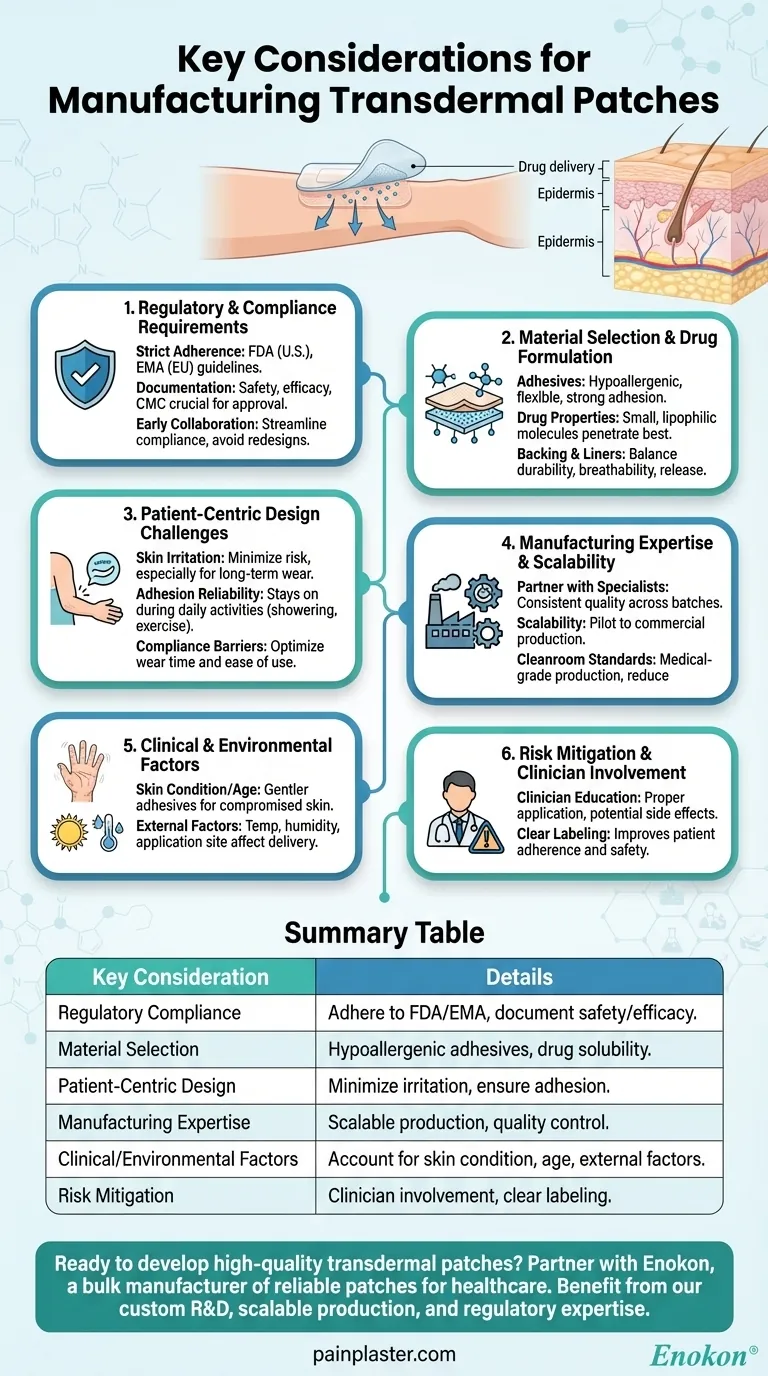
When manufacturing transdermal patches, key considerations include regulatory compliance, material selection, patient-specific factors, and production expertise. The patch must adhere to medical standards while using skin-safe adhesives and accounting for drug properties like molecular size and polarity. Challenges such as skin irritation, adhesion reliability, and patient compliance must be addressed through design and clinician collaboration. Partnering with experienced manufacturers ensures scalability, quality, and regulatory adherence from development to market.

Key Points Explained:
1. Regulatory and Compliance Requirements
- Transdermal patches are classified as medical devices or drug-delivery systems, requiring strict adherence to regulations like FDA (U.S.) or EMA (EU) guidelines.
- Documentation of safety, efficacy, and quality control (e.g., Chemistry, Manufacturing, and Controls [CMC]) is critical for approval.
- Early collaboration with regulatory experts and experienced converters can streamline compliance and avoid costly redesigns.
2. Material Selection and Drug Formulation
- Adhesives: Must be hypoallergenic, flexible, and maintain adhesion across skin types and conditions (e.g., sweating).
- Drug Properties: Smaller, lipophilic molecules penetrate skin more effectively; solubility and stability in the patch matrix are vital.
- Backing and Release Liners: Materials must balance durability, breathability, and drug-release kinetics.
3. Patient-Centric Design Challenges
- Skin Irritation: Patch materials and drug formulations should minimize irritation risks, especially for long-term wear.
- Adhesion Reliability: Patches must stay intact during daily activities (e.g., showering, exercise) without causing discomfort.
- Compliance Barriers: Design should address patient habits (e.g., forgetfulness) by optimizing wear time and ease of use.
4. Manufacturing Expertise and Scalability
- Partnering with specialized manufacturers ensures:
- Consistent quality across batches.
- Scalability from pilot to commercial production.
- Robust analytical testing (e.g., dissolution rates, stability).
- Expertise in medical-grade production (e.g., cleanroom standards) reduces contamination risks.
5. Clinical and Environmental Factors
- Skin Condition/Age: Elderly or compromised skin may require gentler adhesives or lower drug concentrations.
- External Factors: Temperature, humidity, and application site (e.g., arm vs. torso) affect drug delivery and adhesion.
6. Risk Mitigation and Clinician Involvement
- Clinicians must educate patients on proper application, potential side effects, and contraindications (e.g., for women with specific health risks).
- Clear labeling and instructions improve adherence and safety.
By addressing these factors holistically, manufacturers can create effective, user-friendly patches that meet regulatory and patient needs. Have you considered how material innovations (e.g., microneedle arrays) might further enhance drug delivery? Such technologies quietly redefine modern therapeutics.
Summary Table:
| Key Consideration | Details |
|---|---|
| Regulatory Compliance | Must adhere to FDA/EMA guidelines, document safety/efficacy, and collaborate with regulatory experts. |
| Material Selection | Hypoallergenic adhesives, drug solubility, and durable backing materials are essential. |
| Patient-Centric Design | Minimize skin irritation, ensure adhesion reliability, and address compliance barriers. |
| Manufacturing Expertise | Partner with specialists for scalable production, quality control, and cleanroom standards. |
| Clinical/Environmental Factors | Account for skin condition, age, and external factors like humidity. |
| Risk Mitigation | Clinician involvement for patient education and clear labeling improves safety. |
Ready to develop high-quality transdermal patches? Partner with Enokon, a bulk manufacturer of reliable transdermal patches and pain plasters for healthcare and pharma distributors. Benefit from our technical expertise in custom R&D, scalable production, and regulatory compliance. Contact us today to discuss your project needs!
Visual Guide
Related Products
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Heating Pain Relief Patches for Menstrual Cramps
- Capsaicin Chili Medicated Pain Relief Patches
- Medical Cooling Gel Patches for Fever Cooling Patches
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
People Also Ask
- Can heat patches be used for fresh injuries? Avoid This Common Mistake for Faster Recovery
- What are the common side effects of using the medicated heat patch? Understanding Risks & Safe Use
- What are the key features of the Deep Heat Pain Relief Back Patch? Get Up to 16 Hours of Drug-Free Relief
- How do Deep Heat Pain Relief Patches provide pain relief? Discover the Drug-Free Mechanism
- How does the Deep Heat Back Patch work? A Drug-Free Solution for Targeted Pain Relief














