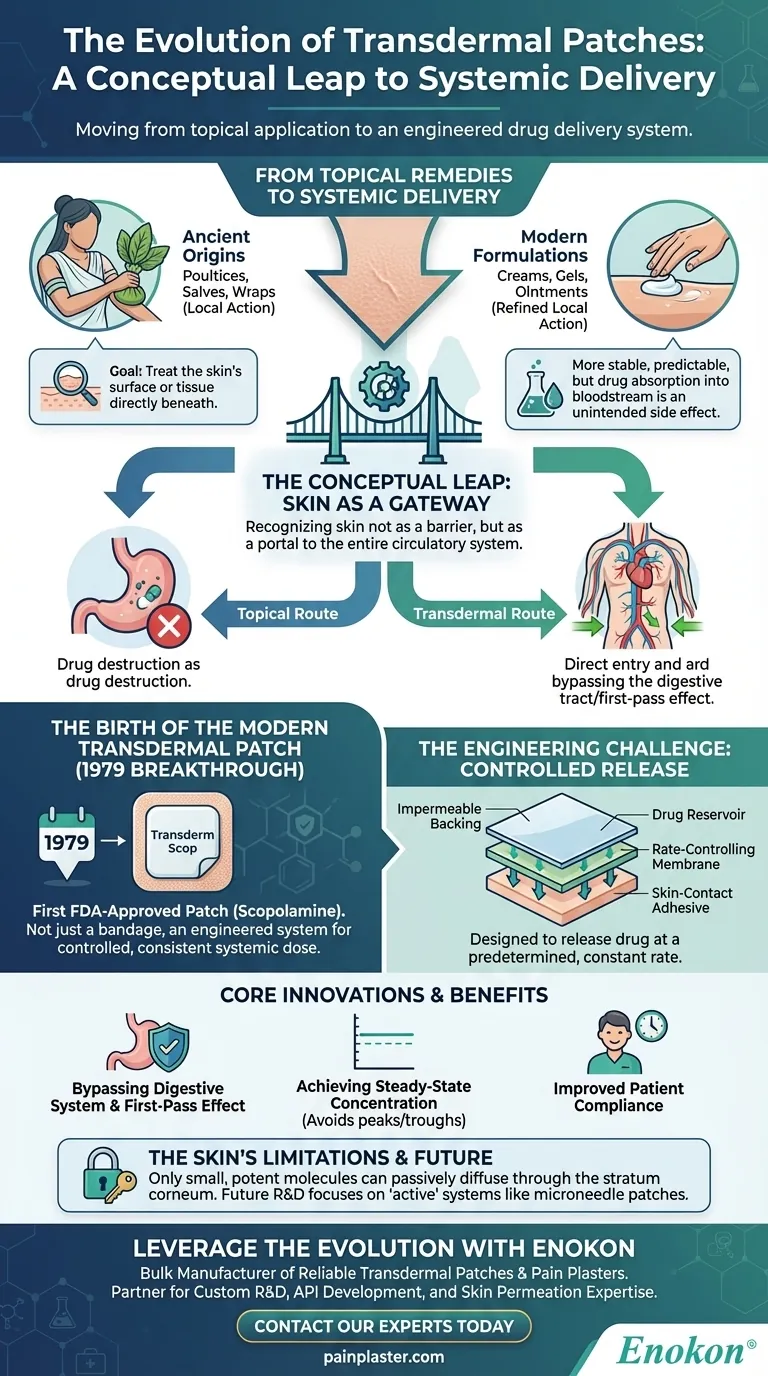
The modern transdermal patch is a direct result of a crucial conceptual leap: moving from using topical applications to treat the skin itself to engineering a system that uses the skin as a controlled gateway for delivering medicine to the entire body. While cultures have applied substances to the skin for millennia, the first true transdermal patch, approved in 1979 for motion sickness, was the first to master systemic, rate-controlled delivery through the skin barrier.
The history of the transdermal patch is not merely an evolution of better creams or ointments. It represents a fundamental shift in scientific understanding—recognizing the skin not just as a protective barrier, but as a viable and strategic organ for drug administration.
From Topical Remedies to Systemic Delivery
The path to the modern patch was paved by a slowly changing perspective on the function of human skin in medicine.
Ancient Origins: Treating the Skin's Surface
For centuries, the goal of applying substances to the skin was purely local. Ancient cultures used poultices, salves, and wraps made from plants and minerals to treat wounds, rashes, and infections directly on the skin's surface.
The therapeutic action was intended for the skin itself or the tissue immediately beneath it. There was no concept of using the skin to treat an internal, systemic condition.
The Rise of Modern Formulations
Over time, pharmacology led to the development of refined topical preparations like creams, gels, and ointments. These offered more stable and predictable local treatments for dermatological issues or muscle pain.
However, the core principle remained the same. These products were designed for local action, with drug absorption into the bloodstream being an unintended, and often minimal, side effect.
The Conceptual Leap: The Skin as a Gateway
The true revolution began when researchers started viewing the skin as a potential portal to the entire circulatory system. They recognized that by delivering a drug through the skin, they could bypass the harsh environment of the digestive tract.
This route avoids the "first-pass effect," where a significant portion of a drug taken orally is destroyed by the liver before it can ever take effect. This insight transformed the skin from a simple target to a strategic delivery pathway.
The Birth of the Modern Transdermal Patch
The idea of using the skin as a gateway required a new technology capable of controlling the drug's release with precision.
The First Breakthrough: Scopolamine in 1979
The first FDA-approved transdermal patch was the Transderm Scop, which delivered scopolamine to prevent motion sickness. This was a landmark achievement.
It wasn't just a medicated bandage; it was an engineered system designed to deliver a specific, consistent dose of medication into the bloodstream over several days. This established the viability of transdermal delivery for a systemic effect.
The Engineering Challenge: Controlled Release
A modern patch is a multi-layered technology. It typically includes an impermeable backing, a reservoir containing the drug, a rate-controlling membrane, and a skin-contact adhesive.
This construction is what differentiates it from a simple cream. The patch is designed to release the drug at a predetermined, constant rate, creating a steady concentration in the blood and avoiding the peaks and troughs associated with oral pills.
Understanding the Core Innovation
The transdermal patch solved several long-standing problems in medicine, but its application is governed by the skin's natural limitations.
Bypassing the Digestive System
The most significant advantage is avoiding the gastrointestinal tract. This protects the drug from stomach acid and liver metabolism, meaning a higher percentage of the drug reaches its target.
Achieving Steady-State Concentration
For many chronic conditions, like pain management or hormone replacement, a stable level of medication is far more effective and has fewer side effects than the sharp spikes from intermittent dosing. Patches excel at providing this steady-state delivery.
The Skin's Limitations
The skin's primary function is to be a barrier. Only certain drugs can be used in a patch. The molecule must be small and potent enough to passively diffuse through the tough outer layer of the skin (the stratum corneum) in a therapeutically effective amount. This is why not all medications are available in patch form.
The Legacy of This Evolution
Understanding this history provides a clear framework for appreciating the role of transdermal technology in modern medicine.
- For understanding medical history: The key innovation was the paradigm shift from treating the skin's surface to viewing the skin as a reliable drug delivery organ.
- For appreciating modern pharmaceuticals: The genius of the patch lies in its engineered system for providing controlled, steady-state drug release over multiple days, improving patient compliance and therapeutic outcomes.
- For future drug development: The limitations of passive diffusion are driving new research into "active" transdermal systems, like microneedle patches, to deliver a wider range of medicines through the skin.
This journey from simple plant poultices to sophisticated drug delivery systems has fundamentally changed how we manage chronic conditions and deliver medicine.
Summary Table:
| Historical Era | Key Development | Core Principle |
|---|---|---|
| Ancient Times | Poultices, Salves, Wraps | Local treatment of skin surface |
| Modern Pharmacology | Creams, Gels, Ointments | Refined local action |
| Conceptual Leap (Mid-20th Century) | Viewing skin as a gateway | Systemic delivery, bypassing digestion |
| 1979 Breakthrough | Transderm Scop Patch | First engineered, rate-controlled systemic delivery |
Leverage the Evolution of Transdermal Technology for Your Brand
As a bulk manufacturer of reliable transdermal patches and pain plasters, Enokon embodies the modern legacy of this medical innovation. We partner with healthcare and pharmaceutical distributors and brands to deliver precisely engineered, controlled-release systems.
Benefit from our technical expertise for custom R&D and development. Whether you need to improve patient compliance for a chronic condition or develop a new delivery system for your API, our team can help you navigate the science of skin permeation and patch design.
Ready to develop your next-generation transdermal product? Contact our experts today to discuss your project requirements.
Visual Guide
Related Products
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Heating Pain Relief Patches for Menstrual Cramps
- Herbal Eye Protection Patch Eye Patch
- Menthol Gel Pain Relief Patch
- Icy Hot Menthol Medicine Pain Relief Patch
People Also Ask
- How does capsaicin work in the medicated heat patch? The Science Behind Pain Relief
- Are heat patches safe for all body parts? Key Safety Zones and No-Go Areas Explained
- What are the key features of the Deep Heat Pain Relief Back Patch? Get Up to 16 Hours of Drug-Free Relief
- What did the UK Million Women Study find regarding transdermal versus oral hormone therapy? A Safer Choice for Gallbladder Health
- Can heat patches be used for fresh injuries? Avoid This Common Mistake for Faster Recovery

















