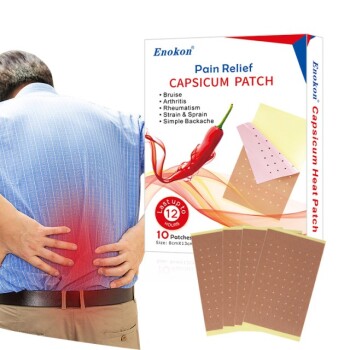
The historical milestone associated with transdermal patches is the FDA approval of the first such patch in 1979, designed to treat motion sickness. This marked the beginning of a new era in drug delivery, enabling controlled, non-invasive administration of medications through the skin. Since then, transdermal patches have evolved significantly, with approvals for diverse medical applications occurring approximately every 2.2 years, including treatments for smoking cessation, birth control, pain relief, and ADHD. This innovation has transformed patient care by offering convenience, sustained release, and improved compliance.

Key Points Explained:
-
First FDA Approval (1979)
- The inaugural transdermal patch was approved to address motion sickness, leveraging scopolamine as the active ingredient.
- This milestone validated the feasibility of transdermal drug delivery, overcoming challenges like skin permeability and dosage control.
-
Impact on Drug Delivery
- Patches eliminated the need for frequent oral dosing or injections, reducing side effects (e.g., gastrointestinal irritation) and improving patient adherence.
- The technology enabled steady drug release over hours or days, optimizing therapeutic efficacy.
-
Expansion of Applications
- Post-1979, patches were developed for nicotine replacement (smoking cessation), hormonal therapies (birth control), opioids (pain management), and stimulants (ADHD).
- Each approval addressed unmet needs, such as minimizing withdrawal symptoms or simplifying contraceptive regimens.
-
Innovation Timeline
- The ~2.2-year approval cycle reflects ongoing advancements in patch design (e.g., matrix vs. reservoir systems) and new active ingredients.
- Modern patches incorporate microneedles or smart sensors for enhanced precision.
-
Patient-Centric Benefits
- Transdermal patches exemplify how incremental innovations can quietly revolutionize daily healthcare routines, from managing chronic pain to quitting smoking.
This trajectory underscores how a single regulatory milestone in 1979 paved the way for transdermal patches to become indispensable in global medicine.
Summary Table:
| Key Milestone | Impact |
|---|---|
| First FDA Approval (1979) | Scopolamine patch for motion sickness validated transdermal drug delivery. |
| Expansion of Applications | Nicotine, hormones, opioids, and stimulants addressed via patches. |
| Patient Benefits | Improved adherence, steady drug release, and reduced side effects. |
| Innovation Cycle | New approvals every ~2.2 years, with advancements like microneedles. |
Partner with Enokon for cutting-edge transdermal solutions
As a trusted bulk manufacturer of transdermal patches and pain plasters, we empower healthcare distributors and brands with reliable, customizable drug delivery systems. Leverage our technical expertise for tailored R&D and scalable production. Contact us today to discuss your project needs!
Visual Guide
Related Products
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Heating Pain Relief Patches for Menstrual Cramps
- Herbal Eye Protection Patch Eye Patch
- Menthol Gel Pain Relief Patch
- Icy Hot Menthol Medicine Pain Relief Patch
People Also Ask
- How does the Deep Heat Back Patch work? A Drug-Free Solution for Targeted Pain Relief
- Are heat patches safe for all body parts? Key Safety Zones and No-Go Areas Explained
- What did the UK Million Women Study find regarding transdermal versus oral hormone therapy? A Safer Choice for Gallbladder Health
- How do Deep Heat Pain Relief Patches provide pain relief? Discover the Drug-Free Mechanism
- What are the key features of the Deep Heat Pain Relief Back Patch? Get Up to 16 Hours of Drug-Free Relief