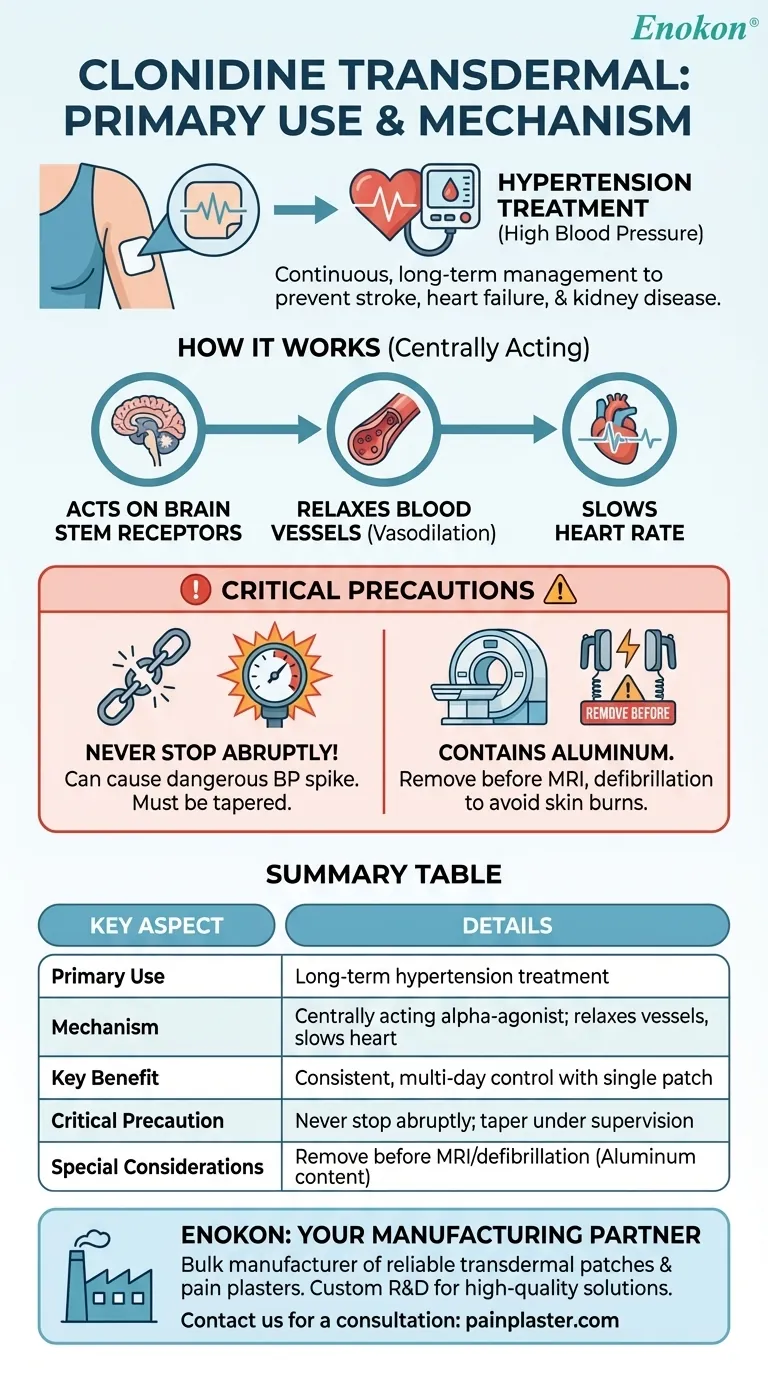
To be clear, clonidine transdermal is a medication patch primarily used, either alone or with other drugs, to treat hypertension, which is the medical term for high blood pressure. It is designed to provide a continuous and steady dose of the medication through the skin to help manage blood pressure over the long term.
The core purpose of clonidine transdermal is not just to lower blood pressure, but to do so consistently over several days with a single patch. This helps prevent the dangerous health outcomes of uncontrolled hypertension, such as stroke, heart failure, and kidney disease.
How Clonidine Transdermal Controls Blood Pressure
Understanding how this medication works is key to appreciating its role in managing hypertension. It operates differently than many other blood pressure medications.
A Centrally Acting Agent
Clonidine belongs to a class of drugs known as centrally acting alpha-agonist hypotensive agents. This means it works by acting on specific receptors in the brain stem.
Relaxing Blood Vessels
By stimulating these receptors, clonidine signals the body to relax the blood vessels. This widening of the vessels, or vasodilation, allows blood to flow more easily and reduces the pressure against the artery walls.
Decreasing Heart Rate
In addition to relaxing blood vessels, the medication also slows the heart rate. A slower, less forceful heartbeat further contributes to the overall reduction in blood pressure.
Key Considerations for Treatment
Using clonidine transdermal effectively requires understanding who it's for and the context of its use.
A Tool for Long-Term Management
High blood pressure is often a chronic condition with no obvious symptoms. Therefore, clonidine is intended for continuous, long-term use to keep blood pressure under control, even if you feel perfectly well.
Standalone or Combination Therapy
Depending on the severity of hypertension and individual patient needs, clonidine transdermal may be prescribed as the sole treatment or in combination with other blood pressure medications.
Special Patient Populations
The safety and effectiveness of this medication have not been established in pediatric patients. Elderly patients may require dose adjustments due to a higher likelihood of age-related heart or kidney problems.
Understanding the Critical Precautions
While effective, clonidine transdermal requires careful management and open communication with your healthcare provider to ensure safety.
The Danger of Abrupt Stoppage
You must never stop using clonidine suddenly. Doing so can cause a rapid and dangerous increase in blood pressure. The medication must be tapered off gradually under a doctor's supervision.
Disclosing Pre-existing Conditions
It is crucial to inform your doctor if you have a history of heart disease, coronary artery disease, heart rhythm disorders, kidney disease, or a history of heart attack or stroke.
Interactions with Medical Procedures
The patch contains aluminum and must be removed before certain medical procedures. It can cause skin burns during an MRI and should also be removed before defibrillation or cardioversion.
Potential for Allergic Reactions
Some individuals may experience an allergic reaction to the patch, such as severe itching or a rash. Any skin reaction at the patch site should be reported to your doctor.
How to Apply This to Your Health
To ensure the best outcome, it's vital to integrate this treatment safely into your life.
If your primary focus is consistent blood pressure control: Use the patch exactly as directed and ensure you always have an adequate supply to avoid missing a dose.
If your primary focus is safety during treatment: Inform every doctor and dentist you see that you are using clonidine and always discuss any upcoming medical procedures.
If your primary focus is managing daily life: Be cautious with alcohol, strenuous exercise, and exposure to heat, as these can increase the drug's effects and lead to dizziness.
Properly managing your medication is a powerful step in taking control of your long-term health.
Summary Table:
| Key Aspect | Details for Clonidine Transdermal |
|---|---|
| Primary Use | Long-term treatment of hypertension (high blood pressure) |
| Mechanism of Action | Centrally acting alpha-agonist; relaxes blood vessels and slows heart rate |
| Key Benefit | Provides consistent, multi-day blood pressure control with a single patch |
| Critical Precaution | Never stop abruptly; must be tapered off under medical supervision to avoid dangerous blood pressure spikes |
| Special Considerations | Contains aluminum; must be removed before MRIs, defibrillation, or cardioversion |
Looking for a reliable manufacturing partner for your transdermal patch needs?
As Enokon, a bulk manufacturer of reliable transdermal patches and pain plasters, we specialize in providing high-quality solutions for healthcare and pharmaceutical distributors and brands. Our technical expertise supports custom R&D and development to bring your specific product vision, like a hypertension patch, to life efficiently and effectively.
Let's discuss how we can support your product line. Contact our experts today for a consultation.
Visual Guide
Related Products
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Heating Pain Relief Patches for Menstrual Cramps
- Herbal Eye Protection Patch Eye Patch
- Asthma Cough and Pain Relief Patch for Adults and Kids
People Also Ask
- What types of pain can the Deep Heat Pain Relief Back Patch be used for? Targeted Relief for Muscles & Joints
- What are the common side effects of using the medicated heat patch? Understanding Risks & Safe Use
- How do Deep Heat Pain Relief Patches provide pain relief? Discover the Drug-Free Mechanism
- What are the key features of the Deep Heat Pain Relief Back Patch? Get Up to 16 Hours of Drug-Free Relief
- How quickly does the Deep Heat Pain Relief Back Patch activate and how long does it provide warmth? Get 16-Hour Relief
















