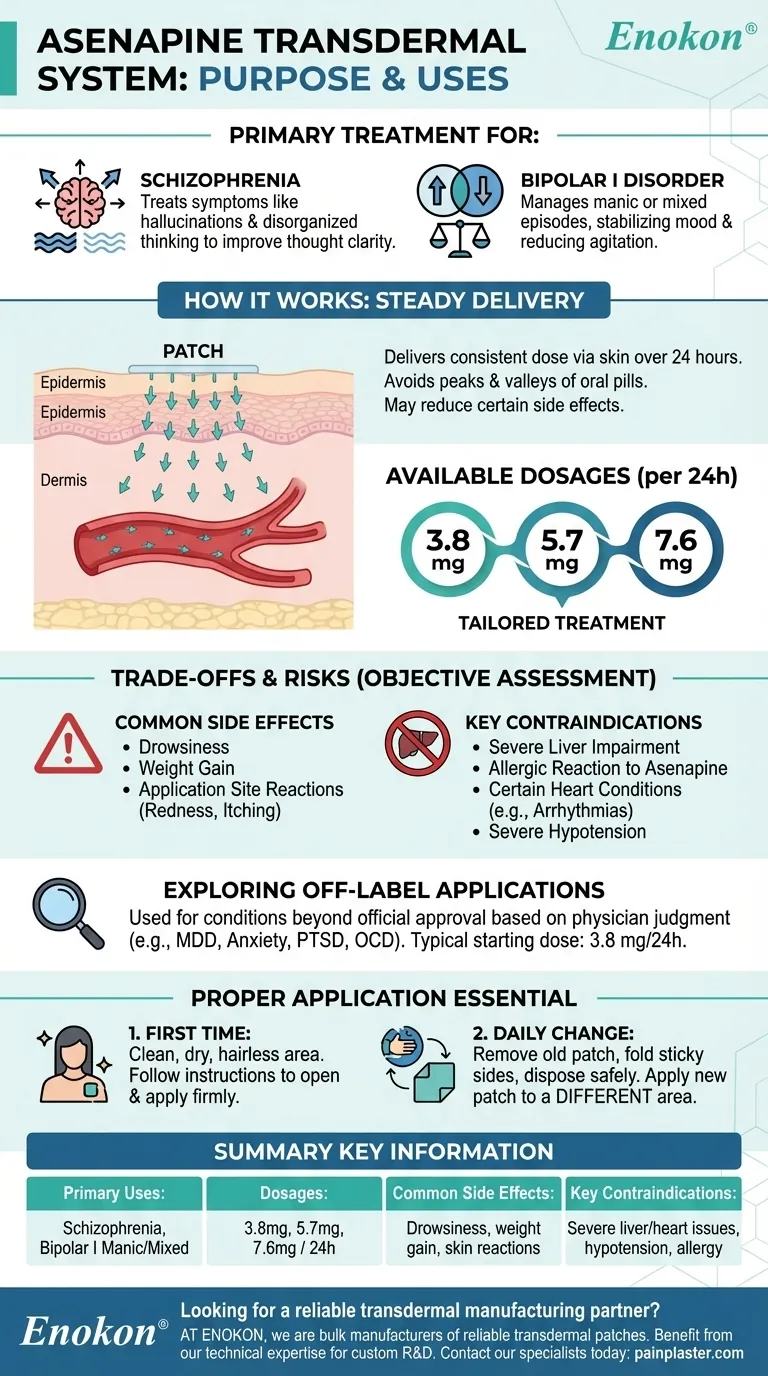
To be clear, the Asenapine transdermal system is a prescription medication primarily used to treat the symptoms of schizophrenia in adults. It is also approved for the treatment of manic or mixed episodes associated with bipolar I disorder in adults. This medication works by helping to restore the balance of certain natural substances in the brain.
The core purpose of the Asenapine transdermal system is to provide a steady, continuous dose of medication through the skin. This delivery method offers an alternative to oral tablets for managing complex mental health conditions like schizophrenia and bipolar disorder.
Understanding the Primary Uses
The Asenapine transdermal system is an atypical antipsychotic specifically formulated to manage severe and persistent mental health conditions. Its approval is targeted at two main disorders.
Treating Schizophrenia
For patients with schizophrenia, this medication helps manage symptoms like hallucinations (seeing or hearing things that are not there) and disorganized thinking. The goal is to improve clarity of thought and daily functioning.
Managing Bipolar I Disorder
In the context of bipolar I disorder, the transdermal system is used to control manic or mixed episodes. It works to stabilize mood, reducing the extreme highs and associated agitation that characterize these periods.
How the Transdermal System Works
A transdermal system, or patch, delivers medication directly through the skin into the bloodstream. This method has specific implications for treatment.
The Advantage of a Patch
The patch provides a steady and consistent release of Asenapine over a 24-hour period. This can help avoid the peaks and valleys in medication levels that can occur with oral pills.
Potential for a Different Side Effect Profile
By delivering the medication steadily, the transdermal system may reduce certain side effects that can be associated with oral formulations. This is a key reason a doctor might choose this delivery method.
Available Dosages
The system is available in three strengths, allowing for tailored treatment based on individual needs. These are 3.8 mg/24 hours, 5.7 mg/24 hours, and 7.6 mg/24 hours.
Understanding the Trade-offs and Risks
Like any powerful medication, the Asenapine transdermal system has potential side effects and is not suitable for everyone. Objective assessment of these risks is critical.
Common Side Effects
Patients may experience side effects such as drowsiness, weight gain, or reactions at the application site. These skin reactions can include redness, itching, or irritation.
Key Contraindications
This medication should not be used by individuals with certain pre-existing conditions. Key contraindications include severe liver impairment, a known allergic reaction to Asenapine, certain pre-existing heart conditions like arrhythmias, or severe hypotension (very low blood pressure).
Exploring Off-Label Applications
In some cases, physicians may prescribe a medication for a condition it is not officially approved to treat. This is known as "off-label" use.
A Note on Off-Label Use
The decision to use a medication off-label is based on a physician's professional judgment that it may be effective for a specific patient's needs.
Potential Conditions
The Asenapine transdermal system has been used off-label to treat a range of other conditions, including major depressive disorder (MDD), anxiety, PTSD, and obsessive-compulsive disorder (OCD). The typical starting dose for such uses is 3.8 mg/24 hours.
How to Apply the Patch Correctly
Proper application is essential for the medication to work effectively and to minimize skin irritation. Follow these steps precisely.
- If you are applying the patch for the first time: Choose a clean, dry, and hairless area of skin, then carefully follow the instructions for opening the pouch and applying the patch firmly.
- If you are changing the patch daily: Remember to remove the old patch, fold it so the sticky sides press together, and dispose of it safely before applying a new patch to a completely different area of skin.
Understanding how to use the Asenapine transdermal system correctly ensures you receive its full therapeutic benefit while managing potential risks.
Summary Table:
| Aspect | Key Information |
|---|---|
| Primary Uses | Schizophrenia in adults; Manic/mixed episodes of Bipolar I Disorder |
| Available Dosages | 3.8 mg/24h, 5.7 mg/24h, 7.6 mg/24h |
| Common Side Effects | Drowsiness, weight gain, skin reactions at application site |
| Key Contraindications | Severe liver impairment, certain heart conditions, severe hypotension, allergy to Asenapine |
Looking for a reliable manufacturing partner for transdermal patches like Asenapine?
At Enokon, we are a bulk manufacturer of reliable transdermal patches and pain plasters. We partner with healthcare and pharmaceutical distributors and brands to bring their medication concepts to life.
Benefit from our technical expertise for custom R&D and development, ensuring a high-quality, effective product for your patients.
Contact our specialists today to discuss your transdermal project needs.
Visual Guide
Related Products
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Capsaicin Chili Medicated Pain Relief Patches
- Heating Pain Relief Patches for Menstrual Cramps
- Asthma Cough and Pain Relief Patch for Adults and Kids
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
People Also Ask
- How do Deep Heat Pain Relief Patches provide pain relief? Discover the Drug-Free Mechanism
- What did the UK Million Women Study find regarding transdermal versus oral hormone therapy? A Safer Choice for Gallbladder Health
- How does capsaicin work in the medicated heat patch? The Science Behind Pain Relief
- Can heat patches be used for fresh injuries? Avoid This Common Mistake for Faster Recovery
- What are the key features of the Deep Heat Pain Relief Back Patch? Get Up to 16 Hours of Drug-Free Relief















