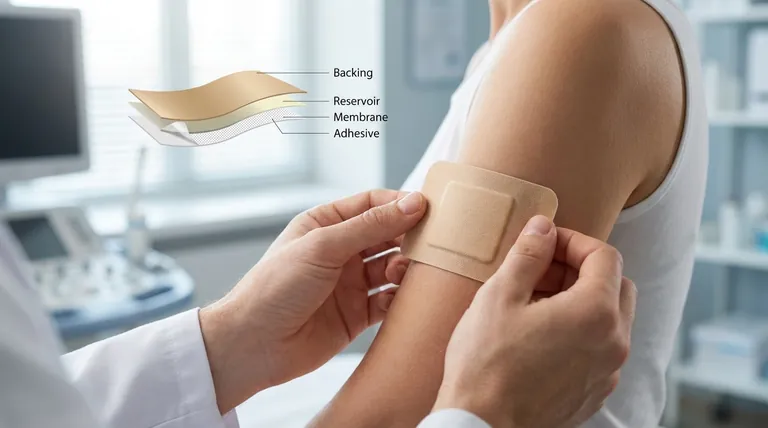
At its core, the clonidine transdermal therapeutic system is a sophisticated, multi-layered adhesive patch designed for controlled medication release. The primary components are a protective backing, a drug reservoir containing clonidine, a rate-controlling membrane, an adhesive layer to secure it to the skin, and a peel-off release liner. This entire structure is typically only 0.2 mm thick.
The essential purpose of the transdermal patch is not just to hold the medication, but to function as a complete system that delivers a steady, controlled dose of clonidine directly through the skin over several days.
Deconstructing the Transdermal System
To understand how the patch works, we must examine the specific function of each layer. Each component plays a critical and distinct role in the controlled delivery of the medication.
The Protective Backing
This is the outermost layer of the patch. Its function is to shield the drug reservoir and other internal components from the external environment, preventing contamination and evaporation.
The Drug Reservoir
This layer contains the active medication, clonidine. It is designed to hold more medication than is needed for the prescribed duration to ensure a consistent delivery rate, which is maintained as long as the reservoir is at least 40% saturated.
The Rate-Controlling Membrane
This is arguably the most critical component for therapeutic effect. This semi-permeable membrane is positioned between the drug reservoir and the skin, precisely regulating the speed at which clonidine can pass through to the adhesive layer.
The Adhesive Layer
This layer serves a dual purpose. Its primary job is to securely affix the entire system to the patient's skin. In many designs, the adhesive also contains a portion of the drug matrix to initiate delivery upon application.
The Release Liner
This is the protective film that covers the adhesive before use. It is impermeable and must be removed and discarded immediately before applying the patch to the skin.
The Principle of Transdermal Delivery
The design of the clonidine patch solves a specific therapeutic problem: the need for stable medication levels to manage chronic conditions like high blood pressure.
Bypassing the Digestive System
By delivering the drug through the skin (transdermally), the system avoids the "first-pass effect," where medication is partially broken down by the liver after being absorbed from the gut. This allows for lower overall doses and more consistent blood concentrations.
A Mechanism for Steady Control
Clonidine is a centrally acting alpha-agonist hypotensive agent. It works by decreasing heart rate and relaxing blood vessels. A patch ensures a continuous, steady release, which helps maintain stable blood pressure without the peaks and troughs associated with oral medication.
Understanding the Key Variables
While effective, the performance of a transdermal system depends on several factors related to its design and its interaction with the body.
The Importance of the Delivery Rate
The rate-controlling membrane is the key to the system's success. Its design ensures that the skin, not the patch, becomes the ultimate rate-limiting factor. This creates a predictable and consistent absorption profile.
The Role of Skin Condition
The patch must be applied to clean, dry, and intact skin. The condition of the outermost layer of skin, the stratum corneum, can impact the rate of drug absorption.
Adhesion is Non-Negotiable
For the patch to work, it must remain in full contact with the skin for the entire prescribed duration, typically seven days. If the edges lift or the patch comes off, medication delivery will be compromised.
Making the Right Choice for Your Goal
Understanding the system's components helps in its proper application and management.
- If your primary focus is consistent blood pressure control: Ensure the patch is applied on schedule and remains fully adhered to the skin for the entire duration to maintain a steady dose.
- If your primary focus is avoiding skin irritation: Rotate the application site with each new patch and ensure the skin is clean and completely dry before application.
- If your primary focus is ensuring efficacy: Never cut a patch, as this will destroy the rate-controlling membrane and lead to an uncontrolled, potentially dangerous release of the drug.
This engineered system transforms a simple adhesive patch into a precise and reliable drug delivery device.
Summary Table:
| Layer | Primary Function |
|---|---|
| Protective Backing | Shields internal components from the environment |
| Drug Reservoir | Holds the active medication (clonidine) |
| Rate-Controlling Membrane | Precisely regulates the speed of drug release |
| Adhesive Layer | Secures the patch to the skin and initiates delivery |
| Release Liner | Protective film removed before application |
Need a reliable manufacturing partner for your transdermal patch?
As Enokon, a bulk manufacturer of reliable transdermal patches and pain plasters, we provide healthcare and pharma distributors and brands with expert technical support for custom R&D and development. Benefit from our expertise to create sophisticated, multi-layered drug delivery systems tailored to your specific active ingredients and release profiles.
Contact our specialists today to discuss your project requirements and how we can support your product development from concept to commercial scale.
Visual Guide
Related Products
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Heating Pain Relief Patches for Menstrual Cramps
- Herbal Eye Protection Patch Eye Patch
- Asthma Cough and Pain Relief Patch for Adults and Kids
People Also Ask
- How does capsaicin work in the medicated heat patch? The Science Behind Pain Relief
- What did the UK Million Women Study find regarding transdermal versus oral hormone therapy? A Safer Choice for Gallbladder Health
- Are heat patches safe for all body parts? Key Safety Zones and No-Go Areas Explained
- How do Deep Heat Pain Relief Patches provide pain relief? Discover the Drug-Free Mechanism
- What are the key features of the Deep Heat Pain Relief Back Patch? Get Up to 16 Hours of Drug-Free Relief














