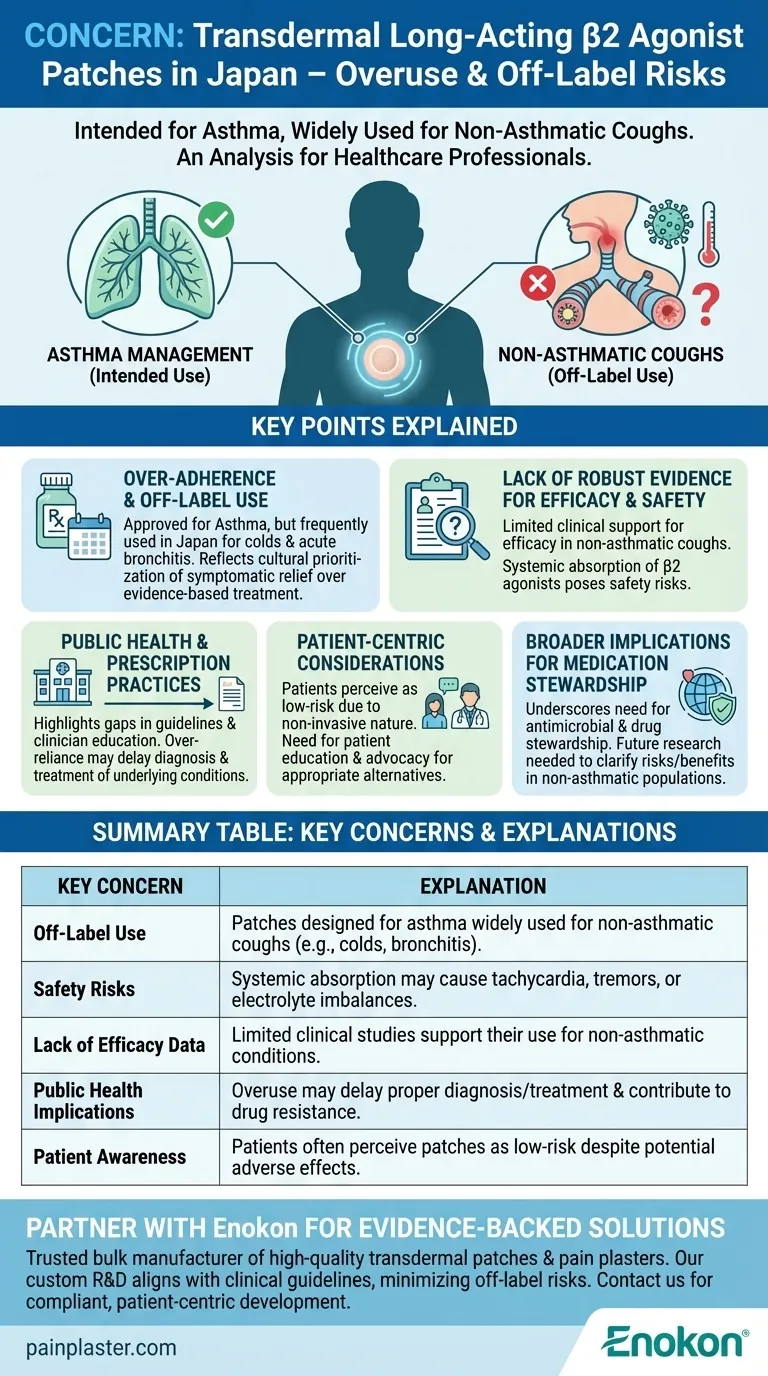
The primary concern regarding the use of transdermal long-acting β2 agonist patches in Japan revolves around their overuse and inappropriate application beyond their intended purpose. These patches, designed primarily for asthma management, are being widely adopted as anti-tussive (cough-suppressing) agents for conditions like acute bronchitis and the common cold, despite limited evidence supporting their efficacy and safety in these contexts. This off-label use raises significant questions about patient safety, potential side effects, and the broader implications of medication adherence without robust clinical justification.

Key Points Explained:
-
Over-Adherence and Off-Label Use
- Transdermal long-acting β2 agonist patches are approved for asthma treatment but are frequently used in Japan to manage coughs associated with non-asthmatic conditions like acute bronchitis or the common cold.
- This practice reflects a cultural or systemic tendency to prioritize symptomatic relief over evidence-based treatment, potentially leading to unnecessary drug exposure.
-
Lack of Robust Evidence for Efficacy and Safety
- The effectiveness of these transdermal patch formulations as anti-tussive agents is not well-supported by clinical studies, particularly for non-asthmatic coughs.
- Safety concerns arise from the systemic absorption of β2 agonists, which may cause adverse effects such as tachycardia, tremors, or electrolyte imbalances when used indiscriminately.
-
Public Health and Prescription Practices
- The widespread off-label use suggests gaps in prescribing guidelines or clinician education, highlighting the need for stricter regulatory oversight or clearer indications.
- Over-reliance on these patches may delay the diagnosis and treatment of underlying conditions (e.g., infections) that require targeted therapies rather than symptomatic suppression.
-
Patient-Centric Considerations
- Patients may perceive the patches as convenient or low-risk due to their non-invasive nature, unaware of potential systemic effects or the lack of evidence for their current use.
- Healthcare providers should prioritize patient education and advocate for alternatives (e.g., non-pharmacological measures or condition-specific medications) when appropriate.
-
Broader Implications for Medication Stewardship
- This scenario underscores the importance of antimicrobial and drug stewardship programs to curb inappropriate medication use, ensuring therapies align with evidence-based guidelines.
- Future research should focus on clarifying the risks and benefits of transdermal β2 agonists in non-asthmatic populations to inform policy and practice.
By addressing these concerns, stakeholders can mitigate risks while preserving the legitimate role of transdermal patches in asthma management. Have you considered how cultural perceptions of medication efficacy might influence such prescribing trends?
Summary Table:
| Key Concern | Explanation |
|---|---|
| Off-Label Use | Patches designed for asthma are widely used for non-asthmatic coughs (e.g., colds, bronchitis) without sufficient evidence. |
| Safety Risks | Systemic absorption may cause tachycardia, tremors, or electrolyte imbalances. |
| Lack of Efficacy Data | Limited clinical studies support their use for non-asthmatic conditions. |
| Public Health Implications | Overuse may delay proper diagnosis/treatment and contribute to drug resistance. |
| Patient Awareness | Patients often perceive patches as low-risk despite potential adverse effects. |
Need reliable, evidence-backed transdermal solutions? Partner with Enokon—a trusted bulk manufacturer of high-quality transdermal patches and pain plasters for healthcare distributors and brands. Our expertise in custom R&D ensures formulations align with clinical guidelines, minimizing off-label risks. Contact us to discuss compliant, patient-centric patch development.
Visual Guide
Related Products
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Capsaicin Chili Medicated Pain Relief Patches
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Menthol Gel Pain Relief Patch
- Icy Hot Menthol Medicine Pain Relief Patch
People Also Ask
- How do Deep Heat Pain Relief Patches provide pain relief? Discover the Drug-Free Mechanism
- Can heat patches be used for fresh injuries? Avoid This Common Mistake for Faster Recovery
- Are heat patches safe for all body parts? Key Safety Zones and No-Go Areas Explained
- What are the common side effects of using the medicated heat patch? Understanding Risks & Safe Use
- What are the key features of the Deep Heat Pain Relief Back Patch? Get Up to 16 Hours of Drug-Free Relief
















