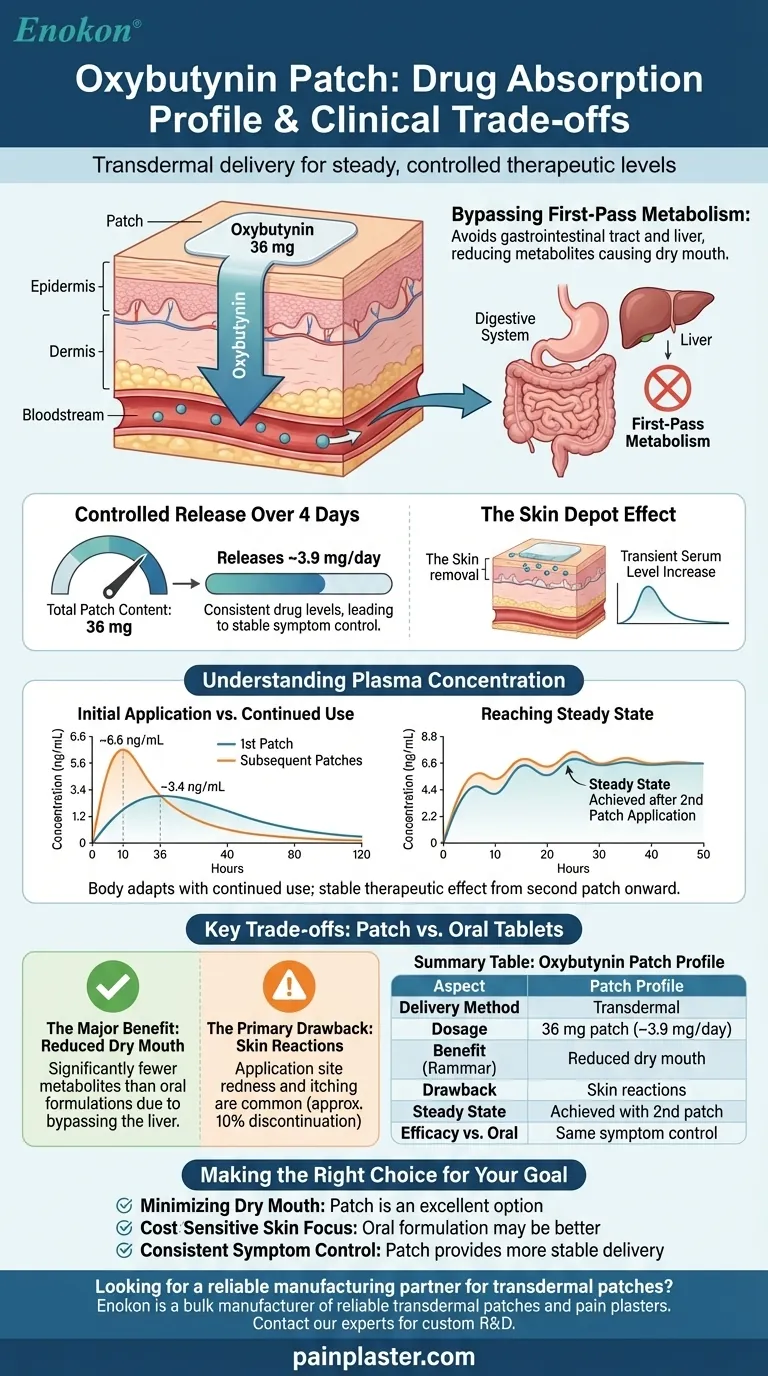
The oxybutynin patch delivers a steady, controlled dose of medication directly through the skin over several days. A standard 39 cm² patch contains 36 mg of oxybutynin and is designed to release an average of 3.9 mg per day. This transdermal method allows the drug to enter the bloodstream directly, achieving stable therapeutic levels after the second application.
The primary advantage of the oxybutynin patch is not superior efficacy but its unique absorption profile. By bypassing the digestive system and liver, it significantly reduces the common and often intolerable side effect of dry mouth, though this benefit comes with the trade-off of potential skin irritation.
How the Patch Delivers Oxybutynin
Bypassing the Digestive System
The transdermal patch adheres to the skin, allowing the medication to permeate its layers and enter the bloodstream directly.
This process of transdermal delivery is crucial because it avoids the gastrointestinal tract and what is known as first-pass metabolism in the liver. This avoidance is the primary reason for its different side-effect profile compared to oral tablets.
A Controlled Release System
The patch is engineered to release the drug at a consistent rate. A patch containing 36 mg of oxybutynin will deliver approximately 15.9 mg over a 96-hour (4-day) period.
This steady release helps maintain consistent drug levels in the body, which can lead to more stable symptom control compared to the peaks and troughs of immediate-release oral medications.
The Skin Depot Effect
After the patch is removed, a small amount of oxybutynin remains in the top layers of the skin.
This "skin depot" can cause a brief, transient increase in serum levels immediately following removal. However, these levels begin to decline within an hour.
Understanding Plasma Concentration Over Time
Initial Application vs. Continued Use
A single, first-time application of the patch results in a peak plasma concentration of approximately 3.4 ng/mL, which is reached at about 36 hours.
With continued use, the body adapts. During subsequent applications, the peak concentration is higher (around 6.6 ng/mL) and is reached much faster, at about 10 hours.
Reaching a Steady State
A steady state, where the amount of drug entering the body equals the amount being eliminated, is achieved during the application of the second patch.
This means a patient will experience the full, stable therapeutic effect from the second patch onward.
Gradual Decline After Peak
Once the peak plasma level is reached during an application cycle, the concentration of oxybutynin in the blood gradually decreases until the patch is removed.
Key Trade-offs: Patch vs. Oral Tablets
The Major Benefit: Reduced Dry Mouth
The most significant clinical advantage of the patch's absorption profile is a lower incidence of dry mouth. Because it bypasses the liver, fewer metabolites that cause this side effect are produced.
The Primary Drawback: Skin Reactions
The direct, prolonged contact with the skin is also the patch's main weakness. Application site reactions, such as redness and itching, are common.
These skin reactions are significant enough to cause approximately 10% of patients to discontinue using the patch.
Efficacy and Cost Considerations
It is important to note that the transdermal patch is no more effective at treating symptoms than either short- or long-acting oral oxybutynin.
Furthermore, the patch typically costs more than the oral formulations.
Making the Right Choice for Your Goal
When deciding on a formulation, the absorption profile has direct consequences for the patient experience.
- If your primary focus is minimizing dry mouth: The patch is an excellent option, as its absorption pathway is specifically designed to reduce this common side effect.
- If your primary focus is cost or you have sensitive skin: An oral formulation may be more appropriate, accepting the higher likelihood of systemic side effects like dry mouth.
- If your primary focus is maintaining consistent symptom control: The patch provides a more stable and continuous drug delivery than immediate-release oral tablets.
Ultimately, understanding the patch's unique absorption pathway is key to balancing its targeted benefits against its specific trade-offs for each individual.
Summary Table:
| Aspect | Oxybutynin Patch Profile |
|---|---|
| Delivery Method | Transdermal (through the skin) |
| Dosage | 36 mg patch, releases ~3.9 mg/day |
| Key Benefit | Significantly reduces dry mouth by avoiding first-pass metabolism |
| Key Drawback | Common application site skin reactions (redness, itching) |
| Steady State | Achieved with the second patch application |
| Efficacy vs. Oral | Same symptom control as oral tablets |
Looking for a reliable manufacturing partner for transdermal patches?
Enokon is a bulk manufacturer of reliable transdermal patches and pain plasters for healthcare and pharmaceutical distributors and brands. Benefit from our technical expertise for custom R&D and development to create effective, patient-preferred drug delivery systems.
Contact our experts today to discuss your project requirements.
Visual Guide
Related Products
- Far Infrared Heat Pain Relief Patches Transdermal Patches
- Far Infrared Deep Heat Relief Patches Medicated Pain Relief Patches
- Asthma Cough and Pain Relief Patch for Adults and Kids
- Heating Pain Relief Patches for Menstrual Cramps
- Capsaicin Chili Medicated Pain Relief Patches
People Also Ask
- How does capsaicin work in the medicated heat patch? The Science Behind Pain Relief
- What did the UK Million Women Study find regarding transdermal versus oral hormone therapy? A Safer Choice for Gallbladder Health
- Can heat patches be used for fresh injuries? Avoid This Common Mistake for Faster Recovery
- How do Deep Heat Pain Relief Patches provide pain relief? Discover the Drug-Free Mechanism
- What are the key features of the Deep Heat Pain Relief Back Patch? Get Up to 16 Hours of Drug-Free Relief
















